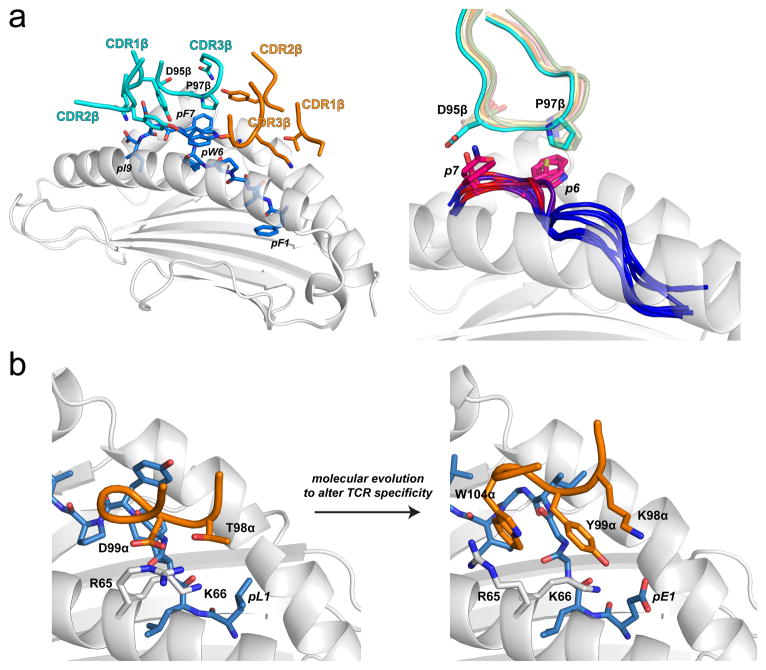
Figure 3.
Hot spots demonstrated by sequence landscapes in TCR-pMHC interfaces. A) Illustration of a structurally conserved hot spot within the interface between the 42F3 TCR and peptides presented by H2-Ld (47). The left panel shows the interface between 42F3 and the QL9 (QLSPFPFDL) mimotope FLSPFWFDI/Ld. The hot spot region is localized to the peptide bulge and engaged primarily by residues of CDR3β. The right panel shows the hot spot as found in eight different agonist 42F3-agonist/Ld structures. Peptide backbones at position 6 through 9 are colored by contact frequency with the TCR, with red indicating the greatest number of contacts. The side chains of the key amino acids at positions 7 and 8 are shown, as is the conformation of the CDR3β loop. Pro97β remains in position to interact with peptide position 6, whereas Asp95β adjusts its conformation to optimize charge complementarity with peptide position 7. B) In altering peptide specificity, the molecular evolution process acted upon a hot spot in the interface between A6 TCR and Tax11-19/HLA-A2 (see also Fig. 1) (38). By changing Thr98α to a lysine and Asp99α to a tyrosine, the complex electrostatic interactions between the TCR and the HLA-A2 α1 helix were disrupted, forcing Arg65 of HLA-A2 to adopt a new conformation, permitting Trp104 of CDR3α to sandwich between the arginine and the peptide backbone and forming a new hot spot in the interface the modified TCR forms with the MART-126-35/HLA-A2 ligand.