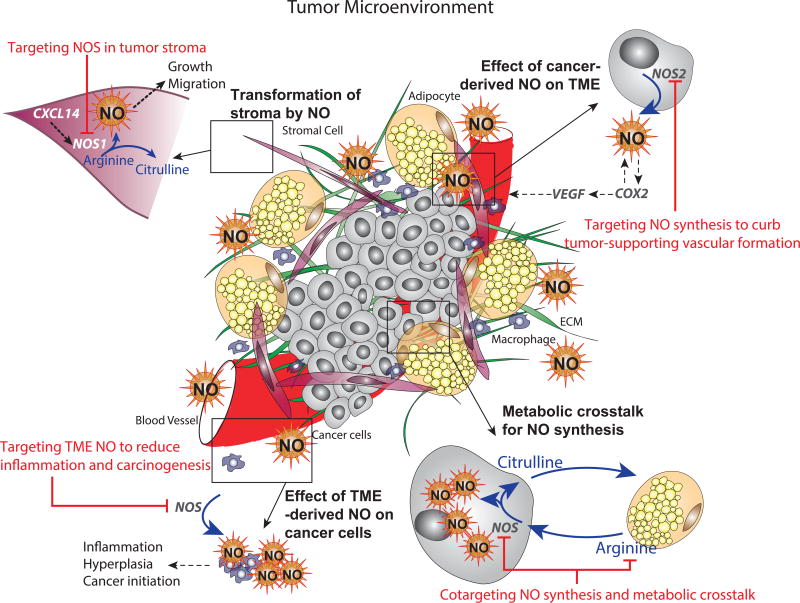
Figure 2. Targeting NOS and NO-mediated pathways in the TME.
Increased NOS expression or NO production in non-malignant stromal compartments are induced by upregulation of chemokine ligands. NO produced by stromal cells contributes to tumor growth and increases migration potential of tumors. Targeting stromal NOS diminishes tumorigenic properties (top left panel).
Cancer cells expressing NOS2 produce NO to induce angiogenesis in tumors by upregulating VEGF. Targeting NOS in cancer cells prevents formation of tumor-supporting blood vessels (top right panel).
O-ASCs in the TME secrete arginine that is utilized by ovarian cancer cells to produce NO via NOS. Citrulline regenerated by cancer cells is utilized by O-ASCs to supplement arginine production. Cotargeting NOS in cancer cells and depleting arginine effectively eliminates metabolic crosstalk in the TME and reduce cancer cell viability (bottom right panel).
Exogenous NO in the TME produced by stromal cells induces inflammation, hyperplasia and initiates tumorigenesis in colorectal cancers. Inhibiting stromal NOS in early stage patients can curb tumor progression (bottom left panel).
NOS, nitric oxide synthase; VEGF, Vascular endothelial growth factor; COX2, Cytochrome oxidase-2; TME, tumor microenvironment; O-ASCs, Omental adipose stellate cells