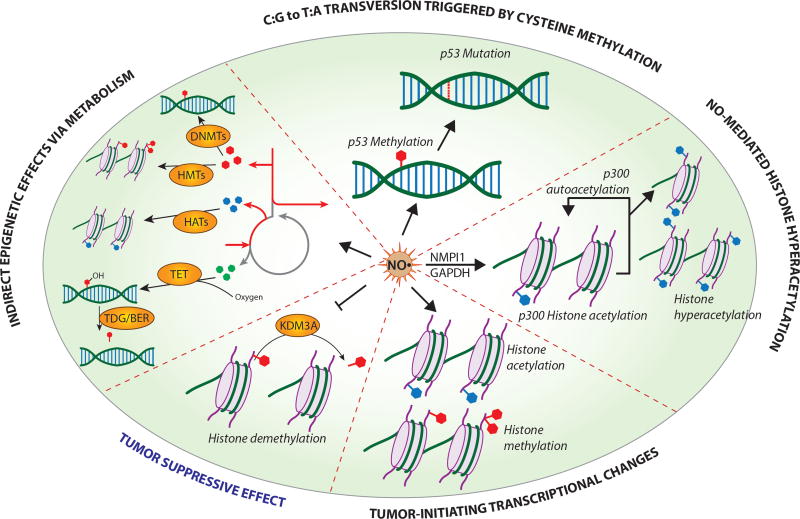
Figure 4. NO regulates epigenetic modifications in tumors.
NO regulates epigenetic modifications via various mechanisms to support tumor progression, or inhibits epigenetic changes that lead to tumorigenesis. NO induces p53 methylation that can lead up to tumor-supporting p53 mutations. NO upregulates NMP1 and GAPDH expression to promote p300 autoacetylation and histone hyperacetylation that is associated with tumor initiation. NO is responsible for genome-wide posttranslational modifications that can lead to oncogenic signatures in breast epithelial cells. NO is speculated to indirectly regulate epigenetics by affecting cellular metabolism that generates cofactors required for epigenetic modifications. Cotargeting NO synthesis and cofactor-producing metabolic reprogramming can provide novel therapeutic approaches. NO suppresses tumor progression by inhibiting KDM3A to reduce histone demethylation known to support tumor growth.
NMP1, Neucleophosmin; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase; KDM3A, Lysine demethylase.