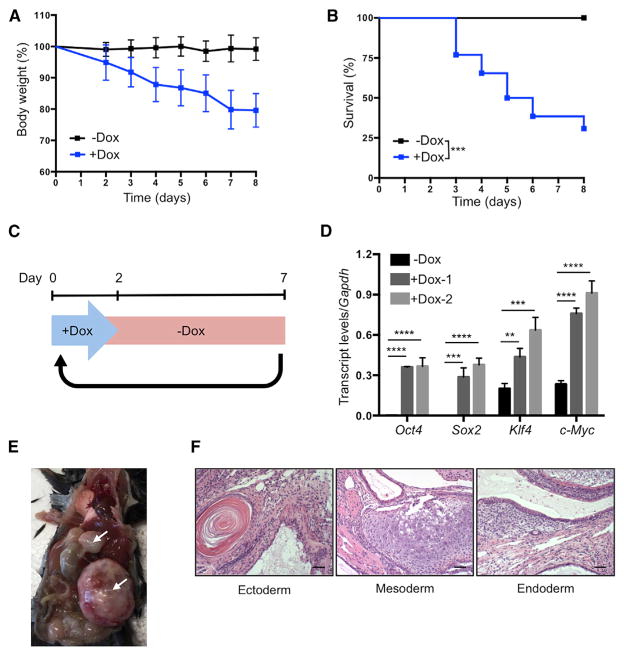
Figure 3. Establishment of In Vivo Induction Protocol in 4F Mice.
(A) Body weight of 4F mice upon continuous administration of doxycycline (−Dox n = 11; +Dox n = 26).
(B) Survival of 4F mice upon continuous administration of doxycycline (−Dox n = 11; +Dox n = 26). ***p < 0.0005 according to log-rank (Mantel-Cox) test.
(C) Schematic representation of cyclic doxycy-cline administration protocol.
(D) qPCR analysis of Oct4, Sox2, Klf4, and c-Myc in blood samples of 4F mice after 2 days of doxycycline administration. **p < 0.005, ***p < 0.0005, and ****p < 0.0001 according to one-way ANOVA with Bonferroni correction.
(E) Teratomas (arrows) in 4F mice carrying two copies of OSKM and rtTA cassette after 8 weeks of cyclic doxycycline administration.
(F) Histological analysis of teratoma with ectoderm, mesoderm, and endoderm. Scale bar, 50 μm.
Data are presented as mean ± SEM. See also Figure S3.