Fig 7. Stimulation of Ssa1 ATPase activity by peptide substrate, but not Ydj1, is reduced in R444E and K446E variants.
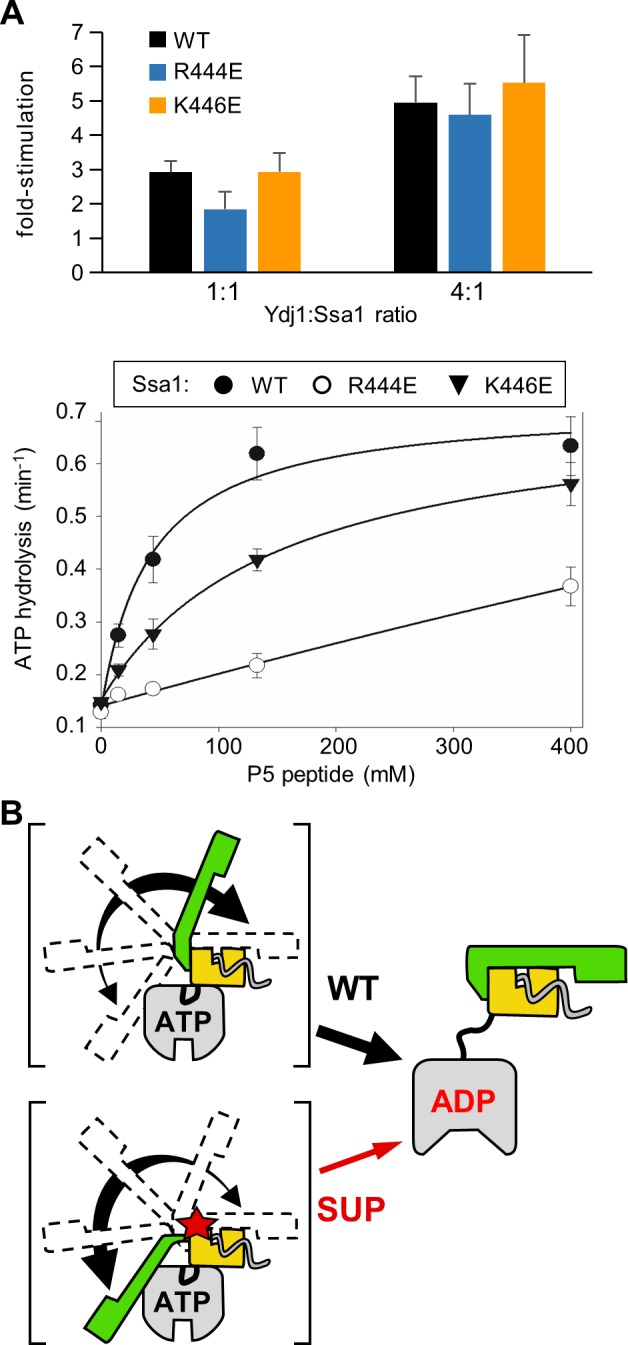
(A) Single turnover ATPase assays were performed using WT and variants Ssa1 32P-ATP complexes. Data are presented as fold-stimulation relative to the basal activity for each Ssa1 protein. Error bars indicate the standard deviation. (top) stimulation by Ydj1. The mean of 6 experiments is plotted. (bottom) Stimulation by peptide P5. Assays were performed at four P5 concentrations and apparent rate constants (min-1) calculated. The mean of three experiments is plotted. Basal turnover rate (min-1): Ssa1, 0.141 +/- 0.003; Ssa1R444E, 0.128; Ssa1K446E, 0.148 +/- 0.007. (B) Schematic representation of the transient intermediates of Hsp70-substrate interaction. Dotted lines in II indicate detachment of SBDα from NBD. Dotted lines in I and III indicate: SBDα transiently detaches from NBD in ATP-state in the absence of substrate interaction (I) and transiently from the SBDβ in the ADP-state (III).
