Abstract
Background
Having an exaggerated reactivity to threats that are uncertain (U-threat) may facil00itate the initiation and maintenance of excessive alcohol use in some individuals. This abnormality may not just be a concomitant for alcohol use disorder (AUD), but also an endophenotype for AUD.
Method
The aim of the current study was therefore to provide a preliminary test of whether U-threat is an endophenotype for AUD using several of the endophenotype criteria outlined by Gottesman and Gould (2003). Specifically, the study examined whether heightened U-threat reactivity is evidenced in those with: 1) current AUD; 2) remitted AUD (early and sustained remission examined separately); and 3) at-risk for AUD by virtue of having a positive family history of AUD. Participants (N=147) completed a well-validated threat-of-shock task and startle eyeblink potentiation was collected as an index of aversive responding. Individuals and all available first-degree family members were diagnosed using structured clinical interviews.
Results
Individuals at-risk for AUD, with current AUD, and AUD in remission (both early and sustained) all displayed exaggerated reactivity to U-threat, but not predictable threat, compared with non-AUD controls. Moreover, there were no significant differences between the at-risk and any of the AUD groups indicating that exaggerated reactivity to U-threat is relatively state-independent and present regardless of current AUD disease status.
Conclusions
The findings together highlight that exaggerated reactivity to U-threat may be a promising endophenotype for AUD that can aid in the refinement of mechanistic AUD disease models.
Keywords: alcohol use disorder, endophenotype, uncertain threat, startle potentiation
1. Introduction
Converging lines of research indicate that exaggerated reactivity to uncertain threat (U-threat) is a core individual difference factor related to multiple forms of psychopathology (Carleton, 2016; Grupe and Nitschke, 2013; Gorka et al., 2017b). Uncertainty about future threats diminishes one’s ability to avoid danger or prepare for its impact, resulting in a generalized feeling of apprehension and hypervigilance, referred to as anticipatory anxiety (Barlow, 2000; Davis et al., 2010). This is in contrast with certain, or predictable threats (P-threat), which are signaled by discrete cues and elicit phasic responses to identifiable threats, referred to as fear (Barlow, 2000; Davis et al., 2010). Although U-threat (and P-threat) is universally aversive, studies show that certain populations find U-threat to be especially anxiety-provoking and display a host of maladaptive cognitive and behavioral responses in the face of uncertain negative events which facilitates and/or maintains psychopathology (Carleton, 2016; Grupe and Nitschke, 2013).
To date, most of the research regarding reactivity to U-threat has been in relation to internalizing psychopathologies, particularly anxiety disorders. However, a few recent studies indicate that individuals who engage in problematic alcohol use also display heightened reactivity to U-threat. In two independent samples, our lab found that greater levels of current problematic alcohol use (e.g., binge drinking) were associated with greater aversive reactivity to U-threat, measured via startle eyeblink potentiation (Gorka et al., 2016a). Recently, Moberg et al. (2017) replicated and extended the findings in Gorka et al. (2016a) by demonstrating that individuals with current alcohol dependence displayed greater startle potentiation to U-threat, but not P-threat, compared with controls. In a separate sample of panic disorder patients, it was similarly shown that individuals with remitted alcohol dependence displayed greater startle potentiation to U-threat, but not P-threat, compared with individuals with no lifetime diagnosis of alcohol use disorder (AUD; Gorka et al., 2013).
Based on prior findings, we have theorized that individuals who are hyper-reactive to U-threat experience chronic anticipatory anxiety and therefore find alcohol intoxication to be negatively reinforcing as alcohol reduces aversive affective states. Indeed, converging evidence 00from the field of neuroscience suggests that exaggerated reactivity to U-threat is mediated by abnormal neural reactivity and functional connectivity within a frontolimbic circuit comprised of the amygdala, bed nucleus of the stria terminalis, anterior insula, orbitofrontal and ventromedial prefrontal cortices, and anterior mid-cingulate cortex (Grupe and Nitschke, 2013). The same frontolimbic circuit is modulated by alcohol consumption (Bjork and Gilman, 2014), and disruptions in functional connectivity within the circuit have recently been shown to contribute to alcohol’s acute subjective anxiolytic effects (Gorka et al., 2017c). Individuals who are highly reactive to U-threat therefore gain reinforcement from alcohol intoxication which may promote ongoing use once drinking is initiated (Baker et al., 2004; Khantzian, 1997; Koob, 2003). Reactivity to U-threat may therefore be a vulnerability factor for problematic alcohol use that precedes AUD onset. In addition, because sensitivity to uncertainty is posited to be a dispositional characteristic that emerges early in life and has neurobiological underpinnings (Shihata et al., 2016; Shankman et al., 2014), exaggerated reactivity to U-threat may be an endophenotype for AUD.
Although multiple definitions of endophenotypes have been put forth (Kendler and Neale, 2010), most definitions suggest that an endophenotype is an intermediate phenotype which links genes to disease processes (Gottesman and Shields, 1973). In their seminal paper, Gottesman and Gould (2003) specified five criteria for a candidate endophenotype: 1) associated with illness; 2) heritable; 3) state-independent; 4) co-segregates with illness within families; and 5) found in higher rates in unaffected relatives of affective individuals than in the general population. In-order for exaggerated reactivity to U-threat to be considered an endophenotype for AUD, it should meet each of these criteria and if so, may be a promising means for identifying the ‘downstream’ traits of AUD and excessive drinking behaviors, as well as the ‘upstream’ consequences of risk-related genetic processes.
There has been some initial evidence to suggest that exaggerated reactivity to U-threat is indeed an endophenotype for AUD. For instance, startle potentiation to U-threat has been shown to be moderately correlated within adult biological siblings (Gorka et al., 2016b). Although it is unclear the extent to which a within (non-twin) sibling correlation reflects genetic or environmental factors, the findings suggest that reactivity to U-threat is potentially heritable (endophenotype criterion #2). In the same sample, data also indicated that independent of an individuals’ own AUD and substance use disorder (SUD) status, greater startle potentiation to U-threat, but not P-threat, was associated with greater first-degree family density/loading of AUD (Gorka et al., 2016b; endophenotype criterion #5). Gorka et al. (2016b) further demonstrated that there was no association between reactivity to U-threat and family history of illicit SUD, suggesting that there may be a particularly unique relation between AUD and reactivity to U-threat. Lastly, as was previously mentioned, individuals with both current and remitted AUD have been shown to display exaggerated reactivity to U-threat, highlighting that individual differences in response to U-threat may be state-independent (endophenotype criterion #3).
Prior studies provide strong initial evidence to suggest that reactivity to U-threat confers risk for AUD and may be a candidate endophenotype; however, several key questions remain. Most notably, the evidence for reactivity to U-threat’s state-independence comes from separate samples, and the only study with remitted alcoholics was exclusively comprised of anxiety disorder patients (Gorka et al., 2013), which limits cross-study comparisons and raises concerns about the extent to which exaggerated reactivity to U-threat in the remitted AUD group was driven by comorbid anxiety. AUD is also characterized by a chronically relapsing and remitting course (Dawson et al., 2005; Moos and Moos, 2006) and there are qualitative as well as neurobiological differences between individuals who are early in their remission relative to sustained abstinence (Bartsch et al., 2007; Dennis et al., 2007). Given the heterogeneity within ‘remitted AUD’ and the importance of length of remission, it is essential to distinguish between early vs. sustained remission when examining reactivity to U-threat. Related, no study has compared individuals at various stages of AUD psychopathology, from risk to remission, and it is unknown the extent to which reactivity to U-threat changes along with changes in drinking behaviors and AUD illness.
The potential for alcohol’s state effects on threat responding is especially salient in the context of AUD given that alcohol exposure is known to disrupt neural systems which mediate affective processes, resulting in exacerbated anxiety and stress-responding during active AUD illness and periods of acute and sustained withdrawal (Koob, 2003; Koob and Le Moal, 2001; Koob and Volkow, 2010). Therefore, even if reactivity to U-threat is elevated in individuals at all stages of AUD psychopathology relative to controls, those with active AUD may exhibit the greatest response to U-threat and have the highest levels of anticipatory anxiety, which maintains drinking behaviors. Exploring the role of disease status is essential for not only testing reactivity to U-threat as an endophenotype but also in shedding light on the mechanisms underlying the progression of AUD illness.
The current study examined whether U-threat meets several of the Gottesman and Gould (2003) criteria for being an endophenotypes for AUD. Specifically, we compared response to U-threat (and P-threat) across five diagnostic groups: 1) current AUD; 2) AUD in early remission (i.e., no AUD symptoms for >3 but <12 months); 3) AUD in sustained remission (i.e., no AUD symptoms for >12 months); 4) at-risk for the development of AUD defined as no personal lifetime diagnosis but a positive first-degree family history of AUD (Kendler et al., 1997); and 5) a sample of controls with no personal or family history of AUD. The inclusion of all five groups within the same study allows for a direct test of whether U-threat is elevated in those with active AUD status (criteria #1), remitted AUD (criteria #3), and unaffected relatives of those with AUD (criteria #5). Notably, given the potential role of prolonged alcohol withdrawal syndrome on threat reactivity (Koob, 2013), we distinguished between early vs. sustained remission rather than collapsing into one remitted AUD group. All participants completed a well-validated threat-of-shock task and startle eyeblink potentiation was collected as an index of aversive responding. Individuals and all available first-degree family members completed a structured clinical interview. We hypothesized that the AUD risk, current AUD, and remitted AUD groups would all display greater startle potentiation to U-threat, but not P-threat, compared with the controls. We also speculated that individuals with current AUD and AUD in early remission would exhibit greater startle to U-threat (only) compared with all other groups.
2. Methods
2.1 Participants
Participants were taken from a larger study examining familial neurobiological processes across a range of internalizing and externalizing diagnoses. Inclusion criteria for the larger family study included being between 18–30 years old and having at least one biological sibling in the same age range that was also willing to participate. At least one additional immediate family member (i.e., mother, father, or a third sibling) was also required to be willing and able to participate in the clinical interview portion of the study only. Exclusion criteria included a personal or family history of mania or psychosis, major medical or neurological illness, inability to read or write English, history of serious head trauma, and left-handedness. Participants were recruited via advertisements posted in the community, local psychiatric clinics, nearby college campuses and in area newspapers/websites. A variety of advertisements were used to target different populations including individuals with current and remitted AUD.
A total of 186 families enrolled in the protocol and had diagnostic information on at least two family members. A total of 39 were excluded from the study due to missing or unusable startle eyeblink data. From the remaining sample of 147 families we created our five diagnostic groups of interest – current AUD (n=20), AUD in early remission (n=23), AUD in sustained remission (n=37), healthy individuals at-risk for AUD (n=22), and controls (n=45). The current and remitted AUD groups were defined based on the Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM-5). Healthy individuals at-risk for AUD were required to have no personal lifetime diagnosis of AUD and at least one first-degree relative with lifetime AUD. Controls had no personal or first-degree family history of AUD. Of note, the aims of the larger study dictated that startle eyeblink data was collected from two siblings per family. For the purposes of the current study, only one individual within the sibling dyad was assigned to one of the five groups (i.e., the proband) and the other was treated as a family member similar to mother, father, and all additional siblings (thus, analyses did not violate the assumption of independence of observation).
Participants provided written informed consent after review of the protocol as approved by the university Institutional Review Board. Probands and family members completed a semi-structured clinical interview (detailed below) and a battery of questionnaires, and probands completed a set of laboratory tasks.
2.2 Assessment of Psychopathology
Lifetime diagnoses of Axis I disorders were assessed via the Structured Clinical Interview for DSM-5 Disorders (SCID-5; American Psychiatric Association, 2015) by trained assessors, supervised by a licensed clinical psychologist. Proband interviews were conducted in person. To increase study feasibility, family members had the option to complete the SCID in person or via telephone, which has been shown to have high inter-method reliability (Rohde et al., 1997).
2.3 Laboratory Procedures and Threat Task
All individuals provided a negative breath alcohol sample upon arrival to the session. Details of the laboratory procedures have been published previously (Gorka et al., 2016a,b) and are briefly noted here. Participants first completed a 2-min startle habituation task during which 6 acoustic probes were administered. Next steps included shock electrode placement on participants’ non-dominant left wrist and a second habituation task to ensure that attachment of the shock electrodes did not significantly re-potentiate early startle. Afterwards, a shock work-up procedure was completed in which participants received increasing levels of shock intensity until they reached a level that they described as “highly annoying but not painful.” Ideographic shock levels were used to ensure equality in perceived shock aversiveness (Rollman and Harris, 1987; max level = 5mA).
Participants completed the threat task modeled after the No-Predictable-Unpredictable (NPU) task developed by Grillon and colleagues (Schmitz and Grillon, 2012), extensively used in our lab (Gorka et al., 2013, 2016a,b; Shankman et al., 2013). The task includes three within-subject conditions - no shock (N), predictable shock (P), and unpredictable shock (U). Text at the bottom of the computer monitor informs participants of the current condition by displaying: “no shock” (N), “shock at 1” (P), or “shock at anytime” (U). Each condition lasted 145-s, during which a 4-s visual countdown (CD) was presented six times. The interstimulus intervals (ISIs; i.e., time between CDs) ranged from 15 to 21-s (M = 18.0-s) during which only the text describing the condition was on the screen. During the N condition, no shocks were delivered. During the P condition, participants received a shock every time the CD reached 1. During the U condition, shocks were administered at any time. Startle probes were presented during the CD (1–2-s following CD onset) and ISI (4–13-s following ISI onset). Each condition was presented two times in a randomized order (counterbalanced). All participants received 24 total electric shocks (12 in P and 12 in U) and 60 total startle probes (20 in each condition).
2.4 Startle Data Collection and Processing
Startle data collection and processing has been published previously (Gorka et al., 2016a,b) and is briefly noted here. Data acquisition used BioSemi Active Two system (BioSemi, Amsterdam, The Netherlands) and PSYLAB (Contact Precision Instruments, London, UK). Acoustic startle probes were 40-ms duration, 103-dB bursts of white noise presented binaurally through headphones. Electric shocks lasted 400-ms and were administered to the wrist of the participants’ left hand.
Startle responses were recorded from two 4-mm Ag/AgCl electrodes placed over the orbicularis oculi muscle below the left eye. The ground electrode was located at the frontal pole (Fpz) of an electroencephalography cap that participants were wearing as part of the larger study. One startle electrode was placed 1-cm below the pupil and the other was placed 1-cm lateral of that electrode. Data were collected using a bandpass filter of DC-500-Hz at a sampling rate of 2000-Hz.
Blinks were processed and scored according to published guidelines (Blumenthal et al., 2005). Data were high-pass filtered (28 Hz), rectified, and then smoothed using a 40 Hz low-pass filter. Peak amplitude of the blink reflex was defined within 20–150-ms following the probe onset relative to baseline. Each peak was identified by software but examined by hand to ensure acceptability. Blinks were scored as non-responses if EMG activity during the 20–150-ms post-stimulus time frame did not produce a blink peak that was visually differentiated from the pre-stimulus baseline activity. Blinks were scored as missing if the baseline period was contaminated with noise, movement artifact, or if a spontaneous or voluntary blink began before minimal onset latency and thus interfered with the startle probe-elicited blink response. Blink magnitude values were used in all analyses.
2.5 Data Analysis Plan
We first tested whether the groups differed in important variables known to influence startle potentiation using a series of planned analyses of variance (ANOVAs) and chi-square tests. Specifically, we examined group differences in age, sex, race/ethnicity, psychiatric medication use, and current and lifetime mood, anxiety, and substance use disorders.
To test our hypotheses, we conducted a repeated measures ANOVA where task condition (3: N, P, U) was specified as a within-subjects variable and group (5: current AUD, AUD early remission, AUD sustained remission, at-risk for AUD, and controls) as a between-subjects variable. Raw startle magnitude during the CD phase of each condition of the task (N, P, U) was used to capture response to the task and match the three conditions on visual stimuli (i.e., a CD was on the screen), consistent with our recent publications (e.g., Gorka et al., 2017c; Gorka et al., 2017a; Gorka et al., 2013). Lifetime SUD (yes/no) was included as a covariate given that the groups differed on rates of SUD (see below). We also included lifetime history of any mood or anxiety disorder (i.e., panic disorder, social anxiety disorder, specific phobia, generalized anxiety disorder, major depressive disorder, dysthymia, and/or posttraumatic stress disorder; yes/no) as a covariate. Although the groups did not differ in rates of depression or anxiety, internalizing psychopathology is known to influence startle reactivity during the NPU task and adjusting for comorbidity is therefore necessary (Gorka et al., 2017b). Any significant condition x group interaction was followed-up by testing the effect of group at each task condition using separate ANOVAs. Significant group effects were then further probed using post-hoc Fisher’s least significant difference (LSD) tests.
3. Results
3.1 Descriptives and Clinical Characteristics
On average, a total of 1.9 ± 0.9 family members of each proband completed the clinical interview. There were no group differences in number of family members enrolled in the study (F[4, 146]=1.38, ns).
Of the individuals in the current AUD group, 75% met criteria for mild AUD, whereas 25% had moderate AUD. For individuals in early remission, 56.5% had mild, 26.1% had moderate, and 17.4% had severe AUD. For individuals in sustained remission, 29.8% had mild, 35.1% had moderate, and 35.1% had severe AUD. The groups did not differ on age, biological sex, or race/ethnicity. Approximately 7.5% of the sample was taking psychiatric medications at the time of assessment and there were no group differences in medication use. With regard to diagnoses, the five groups did not differ on current and lifetime major depressive disorder and all individual anxiety disorders (see Table 1). The only difference between groups was for current (χ2[4] = 11.33, p< .05) and lifetime illicit SUD (χ2[4] = 21.42, p< .05) such that control group had significantly lower rates of SUD than each of the alcohol groups, and individuals with current AUD had significantly greater rates than all other groups.
Table 1.
Demographics and clinical characteristics
| Current AUD (n=20) | AUD Early Remission (n=23) | AUD Sustained Remission (n=37) | At-Risk for AUD (n=22) | Controls (n=45) | ||
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age (years) | 23.4 (3.6) | 22.5 (2.4) | 23.8 (2.7) | 22.0 (3.1) | 22.0 (3.3) | |
| Sex (% female) | 55.0% | 43.5% | 48.6% | 63.6% | 66.7% | |
| Race/Ethnicity | ||||||
| Caucasian | 55.0% | 47.8% | 51.4% | 40.9% | 33.4% | |
| African American | 10.0% | 8.7% | 13.5% | 27.3% | 11.1% | |
| Hispanic | 24.4% | 21.7% | 21.6% | 13.6% | 24.4% | |
| Asian | 5.0% | 17.4% | 5.4% | 4.8% | 22.2% | |
| Other/Biracial | 5.6% | 4.4% | 8.1% | 13.4% | 8.9% | |
| Substance Abuse | ||||||
| Alcohol Binges in Past Month | 5.0 (4.2) | 1.0 (2.2) | 1.0 (2.8) | 0.9 (1.4) | 0.2 (0.8) | |
| Drinks Per Week in Past Month | 13.5 (9.2) | 5.0 (3.7) | 5.1 (5.8) | 2.4 (2.5) | 1.3 (2.5) | |
| Daily Cigarette Smoker | 25.0% | 13.0% | 10.8% | 9.1% | 6.7% | |
| Used Illicit Drugs in Past Month (yes/no) | 60.0% | 39.1% | 29.7% | 18.2% | 8.9% | |
| No. Times Used Cannabis in Past Month | 12.1 (23.5) | 5.5 (9.0) | 3.0 (7.0) | 1.5 (6.0) | 0.5 (1.4) | |
| No. Times Used Drug Other than Cannabis in Past Month | 0.4 (1.1) | 0.5 (2.0) | 0.9 (5.1) | 0.6 (2.9) | 0.0 (0.0) | |
| Comorbid Diagnoses and Meds | ||||||
| Current SUD | 25.0% | 13.0% | 10.8% | 4.5% | 6.7% | |
| Lifetime SUD | 60.0% | 40.0% | 35.1% | 18.2% | 8.9% | |
| Current MDD | 0.0% | 4.3% | 2.7% | 0.0% | 2.2% | |
| Lifetime MDD | 40.0% | 43.5% | 37.8% | 36.4% | 26.7% | |
| Current Panic Disorder | 5.0% | 8.7% | 2.7% | 0.0% | 2.2% | |
| Lifetime Panic Disorder | 5.0% | 13.0% | 8.1% | 0.0% | 6.7% | |
| Current SAD | 10.0% | 4.3% | 13.5% | 9.1% | 11.1% | |
| Lifetime SAD | 20.0% | 17.4% | 29.7% | 18.2% | 22.2% | |
| Current Specific Phobia | 25.0% | 13.0% | 16.2% | 13.6% | 15.6% | |
| Lifetime Specific Phobia | 30.0% | 21.7% | 24.3% | 18.2% | 24.4% | |
| Current PTSD | 0.0% | 4.3% | 0.0% | 0.0% | 2.2% | |
| Lifetime PTSD | 15.0% | 8.7% | 13.5% | 4.5% | 4.4% | |
| Current GAD | 0.0% | 8.6% | 0.0% | 0.0% | 4.4% | |
| Lifetime GAD | 0.0% | 13.0% | 10.8% | 0.0% | 11.1% | |
| Taking Psychotropic Meds | 10.0% | 13.0% | 8.1% | 4.5% | 4.4% | |
|
| ||||||
| Startle Magnitude | Variable Range | |||||
| NCD | 71.2 (60.0) | 58.6 (52.6) | 66.1 (73.2) | 73.7 (54.9) | 40.5 (30.6) | 1.8 – 660.5 |
| NISI | 62.1 (48.8) | 50.0 (38.1) | 68.3 (81.3) | 66.0 (50.3) | 39.0 (22.9) | 1.0 – 724.5 |
| PCD | 76.9 (55.3) | 72.4 (71.9) | 82.4 (76.5) | 89.4 (58.7) | 51.5 (47.5) | 1.5 – 973.0 |
| PISI | 57.0 (45.6) | 45.5 (39.4) | 65.7 (67.9) | 68.9 (49.4) | 38.5 (34.6) | 1.2 – 702.8 |
| UCD | 103.6 (69.6) | 102.0 (91.2) | 110.9 (105.2) | 123.1 (72.8) | 56.7 (38.9) | 1.1 –985.3 |
| UISI | 101.2 (65.9) | 97.6 (95.0) | 103.8 (96.3) | 120.4 (70.4) | 64.3 (52.6) | 2.1 – 759.7 |
Note. SUD = any illicit substance use disorder; MDD = major depressive disorder; SAD = social anxiety disorder; PTSD = posttraumatic stress disorder; GAD = generalized anxiety disorder; N = no-threat; P = predictable threat; U = unpredictable threat; ISI = interstimulus interval; CD = countdown.
3.2 Group Differences
Results of the repeated measures ANOVA are presented in Table 2. There was a main effect of task condition such that startle magnitude was greater during U-threat compared with P-threat (p< .001), and greater during both threat conditions compared with no-threat (ps< .001). Individuals with a lifetime history of an internalizing disorder exhibited greater startle magnitude during the task compared with individuals without a history of an internalizing disorder. Results also indicated a significant main effect of AUD group that was qualified by a task condition x AUD group interaction. There was no main effect of lifetime SUD and no other significant interactions.
Table 2.
Results of the omnibus repeated measures ANOVA assessing startle magnitude during the threat task.
| F | df | p-value | np2 | |
|---|---|---|---|---|
| Task Condition* | 30.33 | 2, 280 | <.01 | .18 |
| Lifetime IP* | 3.94 | 1, 140 | .04 | .03 |
| Lifetime SUD | .41 | 1, 140 | .53 | <.01 |
| AUD Group* | 2.98 | 4, 140 | .02 | .08 |
| Condition x Lifetime IP | 2.35 | 2, 280 | .10 | .02 |
| Condition x Lifetime SUD | 1.09 | 2, 280 | .34 | .01 |
| Condition x AUD Group* | 2.99 | 8, 280 | .04 | .06 |
Note.
p < .05;
IP = internalizing psychopathology (i.e., panic disorder, social anxiety disorder, specific phobia, generalized anxiety disorder, major depressive disorder, dysthymia, and/or post-traumatic stress disorder); SUD = substance use disorder.
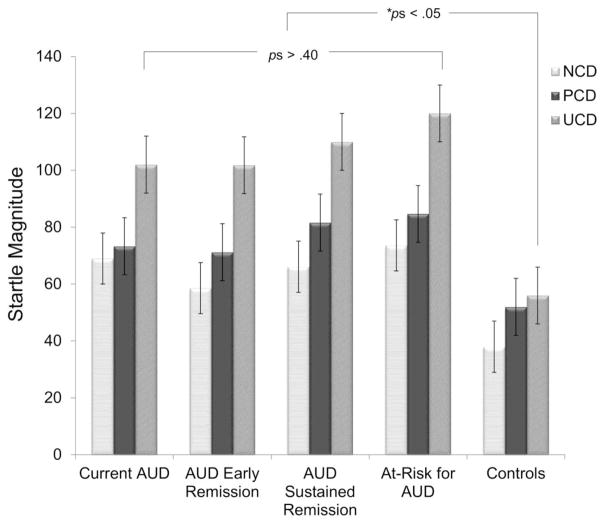
Follow-up analyses for the condition x AUD group interaction revealed that the groups differed during U-threat (F[4, 146] = 3.69, p < .05, np2 = .10), but not during P-threat (F[4, 146] = 1.92, ns, np2 = .05) or no-threat (F[4, 146] = 2.19, ns, np2 = .05). Post-hoc paired comparisons during U-threat are presented in Table 3. The controls displayed significantly lower startle magnitude relative to the current AUD (Cohen’s d=3.2), AUD in early remission (Cohen’s d=3.3), AUD in sustained remission (Cohen’s d=4.6), and at-risk for AUD (Cohen’s d = 4.7) groups1. There were no significant differences amongst any of the four AUD groups (see Figure 1).
Table 3.
Pairwise comparisons between groups during the U-threat condition.
| Mean Difference | |||||
|---|---|---|---|---|---|
|
| |||||
| 1. | 2. | 3. | 4. | 5. | |
| 1. Current AUD | -- | .42 | −8.93 | −18.92 | 45.27* |
| 2. AUD in Early Remission | -- | −9.35 | −19.34 | 44.85* | |
| 3. AUD in Sustained Remission | -- | −9.99 | 54.20* | ||
| 4. At-Risk for AUD | -- | 64.19* | |||
| 5. Controls | -- | ||||
Note.
p < .05.
Means are estimated means adjusted for any lifetime internalizing disorder and lifetime substance use disorder.
Figure 1.
Mean startle magnitude during each condition of the threat task by diagnostic group. NCD = no-threat countdown; PCD = predictable countdown; UCD = unpredictable countdown. Means are adjusted for lifetime diagnoses of substance use disorder and any mood or anxiety disorder. Bars reflect standard error.
4. Discussion
Exaggerated reactivity to U-threat is considered a potential endophenotype for AUD; however, no prior study has attempted to test multiple Gottesman and Gould (2003) endophenotype criteria in the same sample. Results indicated that individuals at-risk for AUD, with current AUD, and AUD in remission (both early and sustained) all displayed exaggerated reactivity to U-threat compared with controls, and the effect was specific to the U-threat condition as there were no group differences observed for P-threat or the no-threat control condition. Moreover, with regards to U-threat, there were no significant differences between the at-risk, current AUD, or remitted AUD groups indicating that exaggerated reactivity to U-threat is relatively state-independent and present regardless of AUD disease status. Our findings together shed important light on the role of reactivity to U-threat in AUD and its potential utility in both research and clinical applications.
The findings suggest that individuals at-risk for the onset of AUD display an exaggerated reactivity to U-threat (Gottesman and Gould [2003] criteria #5), which is present during periods of active AUD status as well as remission (criteria #1 and #3). Notably, our results converge with our prior studies demonstrating that reactivity to U-threat runs in families (criteria #2) and relates to family density of AUD (i.e., percent of family members with AUD, criteria #4; Gorka et al., 2016b). In other words, the present and our prior results together suggest that U-threat may meet the criteria for being an endophenotype for AUD; although further research validating the role of reactivity to U-threat in the onset and maintenance of AUD, specifically, is still needed.
Endophenotypes are purported to be less complex and heterogenous than the associated disorder (i.e., AUD) and therefore, reactivity to U-threat may be determined by fewer genes and reflect an underlying genetic pathway that is more amenable to study than drinking behaviors and AUD symptoms (Walters and Owen, 2007; although see Flint and Munafo, 2007). Along these lines, we purpose that reactivity to U-threat could be considered as a research tool within the alcohol field to study both the clinical traits of AUD as well the neurobiological mechanisms which influence risk and ultimately give rise to the AUD diagnosis. By doing so, we may more accurately and efficiently hone in on a molecular-brain-behavioral based model of AUD that can serve as an objective prevention and intervention target.
Related to above, the results are consistent with theory regarding the role of individual differences in response to U-threat in the onset of AUD. As previously discussed, it is theorized that a subset of individuals is particularly sensitive to uncertain negative events and as a result, experience chronic anticipatory anxiety (Carleton, 2016; Grupe and Nitschke, 2013). Due to ongoing aversive affect, high U-threat reactors likely find acute alcohol intoxication to be reinforcing, which has been strongly supported by several alcohol administration laboratory studies. Indeed, in a series of experiments, Curtin and colleagues and have shown that acute alcohol intoxication effectively, and selectively, dampens startle potentiation to U-threat, but not P-threat, and therefore ameliorates anticipatory anxiety (Bradford et al., 2013; Hefner and Curtin, 2012; Moberg and Curtin, 2009). The subjective and objective relief gained from intoxication likely contributes to the expectancy that alcohol will be stress-dampening and can promote continued use as a means of avoidance-based coping (Oei and Baldwin, 1994). Over time, positive alcohol expectancies and coping-related motives are known to exacerbate drinking behaviors and precipitate the onset of AUD (Baker et al., 2004; Kassel et al., 2000; Patrick et al., 2009). Exaggerated reactivity to U-threat may therefore play a mechanistic role in the initial development of AUD and may serve as a valuable clinical index. Given that reactivity to U-threat can be easily and reliably measured using the NPU paradigm it may be possible to one day screen large groups of adolescents and enroll high U-threat reactors into targeted prevention efforts before the onset of AUD. It may also be useful to screen individuals with current or remitted AUD and administer secondary and tertiary prevention approaches that focus on coping with uncertainty and chronic anticipatory anxiety.
Our initial hypothesis that individuals with current AUD, and AUD in early remission, would display greater reactivity to U-threat compared with the other AUD groups was not supported. The basis for this hypothesis stems from studies indicating that individuals with current AUD exhibit elevated levels of state anxiety and stress reactivity (Sinha, 2007; Sinha et al., 2009). It is possible that U-threat reactivity does not entirely overlap with state anxiety and/or general stress reactivity and reflects a more specific individual difference factor. It is also possible that the lack of state-dependent effects is due to characteristics of the sample. The majority of individuals in the current AUD group had mild AUD, defined as 2–3 symptoms, and all were non-treatment seeking. Individuals with active AUD were also willing and medically able to abstain from alcohol use for the duration of the study visit suggesting that they did not experience significantly impairing acute withdrawal, which is known to exacerbate anxiety (Koob, 2013, 2017). It is therefore plausible that individuals with current, severe AUD and/or patients in acute withdrawal, exhibit especially pronounced levels of reactivity to U-threat which may contribute to cravings and alcohol-seeking behaviors. Exploring the association between severity of current AUD, withdrawal status, and reactivity to U-threat therefore reflects an important line of future research.
The current study focused on AUD, specifically, rather than SUD due to the low rates of illicit SUD in the overall sample and our prior findings that reactivity to U-threat is more related to risk for AUD than SUD (Gorka et al., 2016b). Our AUD groups, however, had higher rates of lifetime SUD and recent substance abuse than the non-AUD groups and although we statistically adjusted for SUD in our analyses, it is possible that the results are not specific to AUD and reflect a broader vulnerability for substance consumption. In fact, other studies have shown that heavy marijuana users (Hefner et al., 2017) and smokers experiencing acute nicotine deprivation (Hogle et al., 2010) display increased startle potentiation to U-threat but not P-threat. It has also recently been argued that heightened reactivity to U-threat reflects a neuroadaptation that emerges as a consequence of drug and alcohol exposure rather than a vulnerability factor (Kaye et al., 2017). Given the characteristics of the sample, it is necessary for future studies to clarify the role of U-threat reactivity in AUD vs. SUD as well as the extent to which excessive anticipatory anxiety is a vulnerability factor vs. an acquired consequence of alcohol/drug exposure. Given that excessive anticipatory anxiety could lead to a host of maladaptive coping responses, it would be beneficial to extend this line of research even beyond substance use to additional deleterious health behaviors (e.g., eating disorders).
The current study had important limitations. First, diagnostic information was only available for family members who completed the clinical interview. Although the interview approach leads to less false-positives by using ‘gold standard’ clinical interviews to evaluate psychopathology (Milne et al., 2009), it leaves the possibility for false-negatives as family members who were not interviewed may have had AUD histories that were not accounted for in the control group. Second, the individual group cell sizes were relatively small, which may have limited our ability to detect important group differences. Third, we used LSD corrections to follow-up our significant findings which is common practice but also a lenient threshold as it does not account for family error rate. When we apply a more stringent correction, such as Bonferroni, not all significant pairwise comparisons survived correction as two became non-significant trends (see footnote 1). Therefore, the present findings should be considered preliminary and require further replication in larger samples. Fourth, there are many variables known to influence both threat reactivity and substance abuse (e.g., genetics, childhood adversity, personality) and we did not measure or account for all possible confounds. Lastly, the sample was drawn from a larger community-based sample and it is unclear whether the findings would generalize to other populations, particularly more severe AUD clinical groups.
In sum, the findings provide preliminary evidence to suggest that exaggerated reactivity to U-threat may be an endophenotype for AUD in that it is relatively state-independent and observed in individuals who are at-risk for AUD as well as in active disease status and remission. Exaggerated reactivity to U-threat could be used to refine mechanistic, neurobiological disease models and identify individuals at-risk for alcohol abuse initiation and escalation. It is worth highlighting that startle potentiation to U-threat is psychometrically reliable and relatively easy to record (Kaye et al., 2016; Lieberman et al., 2017), and is more cost-effective than certain other candidate endophenotypes such as neuroimaging profiles. Studies should therefore continue to test the role of reactivity to U-threat in the onset and maintenance of AUD and begin to elucidate the biologically-mediated pathway that may underlie this individual difference factor.
Highlights.
Exaggerated reactivity to uncertain threat may be an endophenotype for alcohol use disorder (AUD).
This was tested by comparing groups of individuals at various stages of AUD.
The at-risk, current and remitted AUD groups all displayed exaggerated reactivity.
Exaggerated reactivity to uncertain threat is present regardless of AUD status.
Reactivity to uncertain threat is a promising endophenotype for AUD.
Acknowledgments
Role of Funding Source
This research was supported by the National Institute of Mental Health of the National Institutes of Health under award number R01MH098093 (PI: Shankman). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
We also tested for significant group differences using Bonferroni correction rather than LSD correction. Results of the Bonferroni correction indicated that controls displayed significantly lower startle magnitude during U-threat relative to the AUD in sustained remission (p = .02) and the at-risk for AUD groups (p = .01). However, the comparisons between the control group and the current AUD group, and the control group and the AUD in early remission group, became trends (p = .10 and .09, respectively).
Contributors
Stewart Shankman was the principal investigator of the study, assisted in data interpretation, and significantly contributed to the editing of the manuscript. Stephanie Gorka provided the initial study rationale, conducted the statistical analyses, and wrote the first draft of the manuscript. Both authors contributed and have approved the final manuscript.
Conflict of Interest
All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Structured clinical interview for DSM-5 (SCID-5) 2015. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. Am Psychol. 2000;55:1247–1263. doi: 10.1037//0003-066x.55.11.1247. [DOI] [PubMed] [Google Scholar]
- Bartsch AJ, Homola G, Biller A, Smith SM, Weijers HG, Wiesbeck GA, Jenkinson M, De Stefano N, Solymosi L, Bendszus M. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain. 2007;130:36–47. doi: 10.1093/brain/awl303. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, Van Boxtel A. Committee report: Guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bradford DE, Shapiro BL, Curtin JJ. How bad could it be? Alcohol dampens stress responses to threat of uncertain intensity. Psychol Sci. 2013;24:2541–2549. doi: 10.1177/0956797613499923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton RN. Into the unknown: A review and synthesis of contemporary models involving uncertainty. J Anxiety Disord. 2016;39:30–43. doi: 10.1016/j.janxdis.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, Chou PS, Huang B, Ruan W. Recovery from DSM-IV alcohol dependence: United States, 2001–2002. Addiction. 2005;100:281–292. doi: 10.1111/j.1360-0443.2004.00964.x. [DOI] [PubMed] [Google Scholar]
- Dennis ML, Foss MA, Scott CK. An eight-year perspective on the relationship between the duration of abstinence and other aspects of recovery. Eval Rev. 2007;31:585–612. doi: 10.1177/0193841X07307771. [DOI] [PubMed] [Google Scholar]
- Flint J, Munafò MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;37:163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Lieberman L, Klumpp H, Kinney KL, Kennedy AE, Ajilore O, Francis J, Duffecy J, Craske MG, Nathan J, Langenecker S, Shankman SA, Phan KL. Reactivity to unpredictable threat as a treatment target for fear-based anxiety disorders. Psychol Med. 2017a;2017:1–11. doi: 10.1017/S0033291717000964. [DOI] [PubMed] [Google Scholar]
- Gorka SM, Lieberman L, Phan KL, Shankman SA. Association between problematic alcohol use and reactivity to uncertain threat in two independent samples. Drug Alcohol Depend. 2016a;164:89–96. doi: 10.1016/j.drugalcdep.2016.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Hee D, Lieberman L, Mittal VA, Phan KL, Shankman SA. Reactivity to uncertain threat as a familial vulnerability factor for alcohol use disorder. Psychol Med. 2016b;46:3349–3358. doi: 10.1017/S0033291716002415. [DOI] [PubMed] [Google Scholar]
- Gorka SM, Lieberman L, Shankman SA, Phan KL. Startle potentiation to uncertain threat as a psychophysiological indicator of fear-based psychopathology: An examination across multiple internalizing disorders. J Abnorm Psychol. 2017b;126:8–18. doi: 10.1037/abn0000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Nelson BD, Shankman SA. Startle response to unpredictable threat in comorbid panic disorder and alcohol dependence. Drug Alcohol Depend. 2013;132:216–222. doi: 10.1016/j.drugalcdep.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka SM, Phan KL, Childs E. Acute calming effects of alcohol are associated with disruption of the salience network. Addict Biol. 2017c doi: 10.1111/adb.12537. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiat. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Shields J. Genetic theorizing and schizophrenia. Brit J Psychiat. 1973;122:15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: An integrated neurobiological and psychological perspective. Nat Rev Neurosci. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner KR, Curtin JJ. Alcohol stress response dampening: Selective reduction of anxiety in the face of uncertain threat. J Psychopharmacol. 2012;26:232–245. doi: 10.1177/0269881111416691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner KR, Starr MJ, Curtin JJ. Increased stress response to threat among heavy marijuana users. 2017 Manuscript in preparation. [Google Scholar]
- Hogle JM, Kaye JT, Curtin JJ. Nicotine withdrawal increases threat-induced anxiety but not fear: Neuroadaptation in human addiction. Biol Psychiatry. 2010;68:719–725. doi: 10.1016/j.biopsych.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye JT, Bradford DE, Curtin JJ. Psychometric properties of startle and corrugator response in NPU, affective picture viewing, and resting state tasks. Psychophysiology. 2016;53:1241–1255. doi: 10.1111/psyp.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye JT, Bradford DE, Magruder KP, Curtin JJ. Probing for neuroadaptations to unpredictable stressors in addiction: Translational methods and emerging evidence. J Stud Alcohol Drugs. 2017;78:353–371. doi: 10.15288/jsad.2017.78.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassel JD, Jackson SI, Unrod M. Generalized expectancies for negative mood regulation and problem drinking among college students. J Stud Alcohol Drugs. 2000;61:332–340. doi: 10.15288/jsa.2000.61.332. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Davis CG, Kessler RC. The familial aggregation of common psychiatric and substance use disorders in the National Comorbidity Survey: A family history study. Brit J Psychiat. 1997;170:541–548. doi: 10.1192/bjp.170.6.541. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC. Endophenotype: A conceptual analysis. Mol Psychiatry. 2010;15:789–797. doi: 10.1038/mp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of substance use disorders: A reconsideration and recent applications. Harvard Rev Psychiat. 1997;4:231–244. doi: 10.3109/10673229709030550. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: Allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF. Negative reinforcement in drug addiction: The darkness within. Curr Opin Neurobiol. 2013;23:559–563. doi: 10.1016/j.conb.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Koob GF. Stress, negative reinforcement and compulsive drug seeking. Alcohol. 2017;60:203. [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman L, Stevens ES, Funkhouser CJ, Weinberg A, Sarapas C, Huggins AA, Shankman SA. How many blinks are necessary for a reliable startle response? A test using the NPU-threat task. Int J Psychophysiol. 2017;114:24–30. doi: 10.1016/j.ijpsycho.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne BJ, Caspi A, Crump R, Poulton R, Rutter M, Sears MR, Moffitt TE. The validity of the family history screen for assessing family history of mental disorders. Am J Medical Genet B. 2009;150:41–49. doi: 10.1002/ajmg.b.30764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg CA, Bradford DE, Kaye JT, Curtin JJ. Increased startle potentiation to unpredictable stressors in alcohol dependence: Possible stress neuroadaptation in humans. J Abnorm Psychol. 2017;126:441–453. doi: 10.1037/abn0000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg CA, Curtin JJ. Alcohol selectively reduces anxiety but not fear: Startle response during unpredictable vs. predictable threat. J Abnorm Psychol. 2009;118:335–347. doi: 10.1037/a0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moos RH, Moos BS. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction. 2006;101:212–222. doi: 10.1111/j.1360-0443.2006.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oei TP, Baldwin AR. Expectancy theory: a two-process model of alcohol use and abuse. J Stud Alcohol. 1994;55:525–534. doi: 10.15288/jsa.1994.55.525. [DOI] [PubMed] [Google Scholar]
- Patrick ME, Wray-Lake L, Finlay AK, Maggs JL. The long arm of expectancies: Adolescent alcohol expectancies predict adult alcohol use. Alcohol Alcohol. 2009;45:17–24. doi: 10.1093/alcalc/agp066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Seeley JR. Comparability of telephone and face-to-face interviews assessing Axis I and II disorders. Am J Psychiatry. 1997;154:1593–1598. doi: 10.1176/ajp.154.11.1593. [DOI] [PubMed] [Google Scholar]
- Rollman GB, Harris G. The detectability, discrimability, and perceived magnitude of painful electrical shock. Percept Psychophys. 1987;42:257–268. doi: 10.3758/bf03203077. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test) Nat Protoc. 2012;7:527–532. doi: 10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Gorka SM, Nelson BD, Fitzgerald DA, Phan KL, O’Daly O. Anterior insula responds to temporally unpredictable aversiveness: An fMRI study. Neuroreport. 2014;25:596. doi: 10.1097/WNR.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Nelson BD, Sarapas C, Robison-Andrew EJ, Campbell ML, Altman SE, McGowan SK, Katz AC, Gorka SM. A psychophysiological investigation of reward and threat sensitivity in individuals with depression and/or panic disorder. J Abnorm Psychol. 2013;122:322–338. doi: 10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. The role of stress in addiction relapse. Curr Psychiat Rep. 2007;9:388–395. doi: 10.1007/s11920-007-0050-6. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shihata S, McEvoy PM, Mullan BA, Carleton RN. Intolerance of uncertainty in emotional disorders: What uncertainties remain? J Anxiety Disord. 2016;41:115–124. doi: 10.1016/j.janxdis.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Walters JTR, Owen MJ. Endophenotypes in psychiatric genetics. Mol Psychiatr. 2007;12:886–886. doi: 10.1038/sj.mp.4002068. [DOI] [PubMed] [Google Scholar]