Table 1. FDA benefit‐risk assessment.
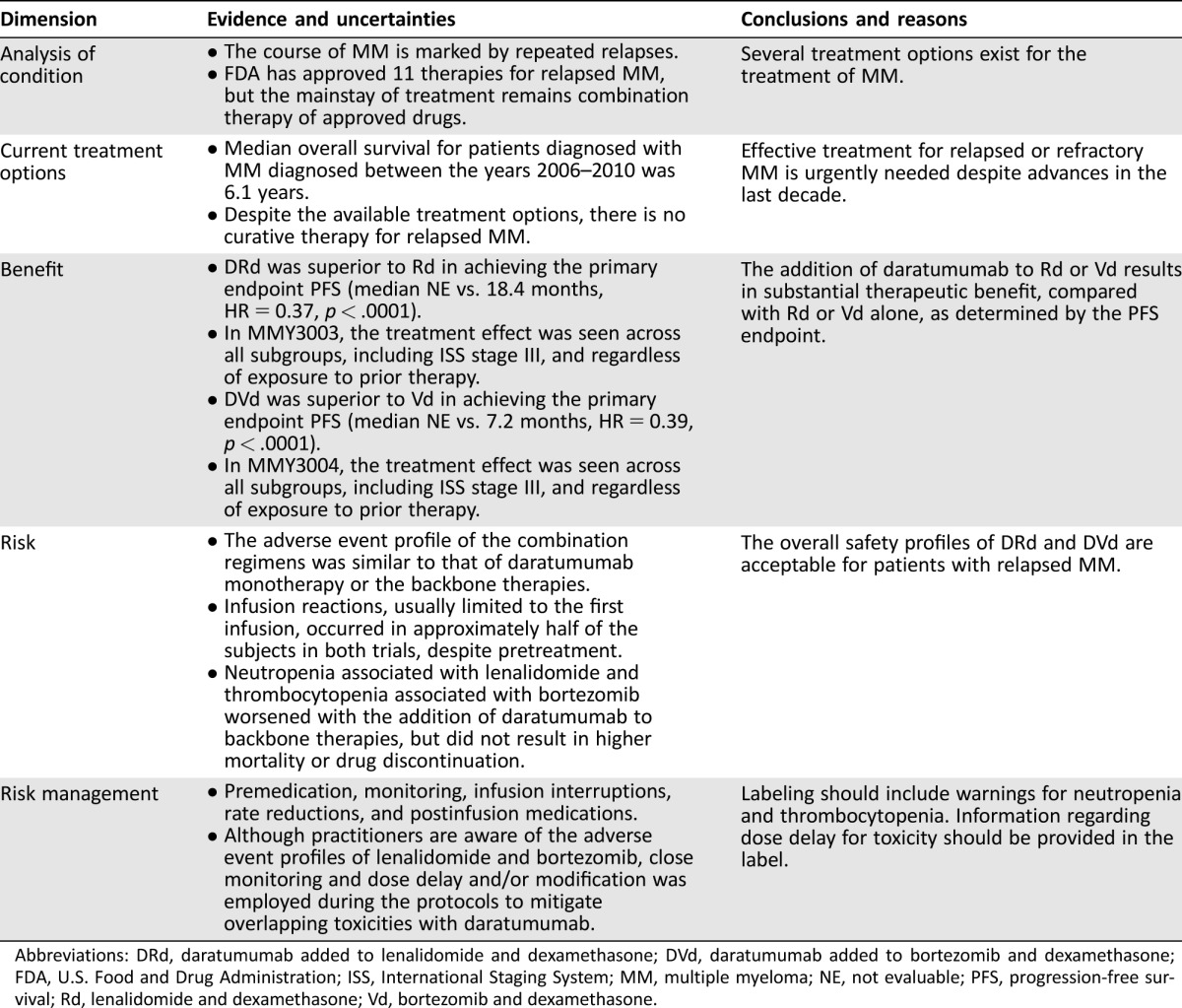
Abbreviations: DRd, daratumumab added to lenalidomide and dexamethasone; DVd, daratumumab added to bortezomib and dexamethasone; FDA, U.S. Food and Drug Administration; ISS, International Staging System; MM, multiple myeloma; NE, not evaluable; PFS, progression‐free survival; Rd, lenalidomide and dexamethasone; Vd, bortezomib and dexamethasone.
