Figure 2. Contributions of FXIIIa to clot biochemical and mechanical stability.
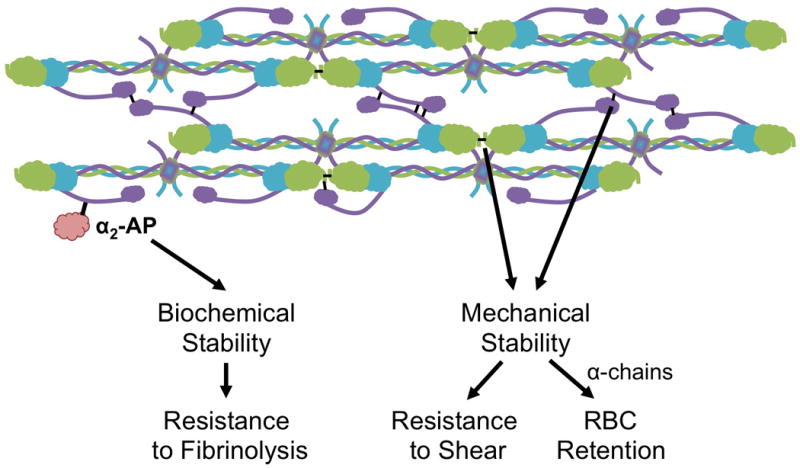
FXIIIa crosslinking of plasma proteins [i.e. α2-antiplasmin, (α2-AP)] increases the resistance of the clot to fibrinolysis. Crosslinking of the fibrin α- (purple) and γ-chains (green) stiffens fibrin fibers and increases the mechanical stability of the clot. Increased mechanical stability renders the clot more resistant to shear forces. α-chain crosslinking enables RBC retention during clot contraction.39
