Summary
Growing evidence points to a deregulated response to Epstein–Barr virus (EBV) in the central nervous system of patients with multiple sclerosis (MS) as a possible cause of disease. We have investigated the response of a subpopulation of effector CD8+ T cells to EBV in 36 healthy donors and in 35 patients with MS in active and inactive disease. We have measured the expression of markers of degranulation, the release of cytokines, cytotoxicity and the regulation of effector functions by inhibitory receptors, such as programmed death 1 (PD‐1) and human inhibitor receptor immunoglobulin‐like transcript 2 (ILT2). We demonstrate that polyfunctional cytotoxic CD8+ CD57+ T cells are able to kill EBV‐infected cells in healthy donors. In contrast, an anergic exhaustion‐like phenotype of CD8+ CD57+ T cells with high expression of PD‐1 was observed in inactive patients with MS compared with active patients with MS or healthy donors. Detection of CD8+ CD57+ T cells in meningeal inflammatory infiltrates from post‐mortem MS tissue confirmed the association of this cell phenotype with the disease pathological process. The overall results suggest that ineffective immune control of EBV in patietns with MS during remission may be one factor preceding and enabling the reactivation of the virus in the central nervous system and may cause exacerbation of the disease.
Keywords: CD8+CD57+ T cells, cytotoxic T‐cell response, Epstein–Barr virus, multiple sclerosis, programmed death 1
Abbreviations
- 7‐AAD
7‐aminoactinomycin B
- B‐LCL
B‐lymphoblastoid cell lines
- CFSE
carboxyfluorescein succinimidyl ester
- CNS
central nervous system
- CSF
cerebrospinal fluid
- EBNA
Epstein–Barr virus nuclear antigen 1
- EBV
Epstein–Barr virus
- GZMB
granzyme B
- IFN‐γ
interferon‐γ
- ILT2/CD85j
human inhibitor receptor immunoglobulin‐like transcript 2
- Iono
ionomycin
- MIP‐1β
macrophage inflammatory protein 1β
- MS
multiple sclerosis
- PBMC
peripheral blood mononuclear cells
- PD‐1
programmed death 1
- PDL‐1
programmed death ligand 1
- PDL‐2
programmed death ligand‐2
- Perf
perforin
- RR‐MS
relapsing–remitting multiple sclerosis
- SP‐MS
secondary progressive multiple sclerosis
- TCR
T‐cell receptor
Introduction
Multiple sclerosis (MS) is an inflammatory and possibly autoimmune disease of the central nervous system (CNS) defined by areas of chronic inflammation with demyelination, axonal damage and loss of neurons.1The cause for the autoreactivity of the immune system against the CNS in patients with MS has been the subject of intensive research. Evidence that individuals who contracted mononucleosis during adolescence show more susceptibility to develop the disease2, 3 suggests that infection by Epstein–Barr virus (EBV) could be a trigger for an autoimmune response in MS. In support of this notion, peripheral blood from patients with MS shows an increased T‐cell response to EBV antigens, especially to EBV nuclear antigen 1 (EBNA1).4, 5, 6 EBV was detected in meningeal tertiary lymphoid structures and in white matter lesions in post‐mortem CNS tissue from patients with MS.7, 8, 9 In addition, in inflamed cerebral meninges of post‐mortem MS brain tissue, infiltrates of effector and cytotoxic CD8+ T cells and innate immune responses characterized by interferon‐α production have been detected in proximity to EBV‐infected plasma cells.8, 10 Therefore, the observed increase of CD8+ T cells specific for EBV lytic antigens in the peripheral blood and cerebrospinal fluid (CSF) of patients with MS during relapses suggests a close association between replication of EBV in the CNS and inflammatory disease activity.11, 12, 13 Also, the evidence for active replication of EBV in patients with MS indicates that the virus has the capacity to escape immune control by cytotoxic lymphocytes.11 However, the results from other studies examining the involvement of EBV infection in the MS pathological process diverged. EBV was undetectable in a heterogeneous B‐cell infiltrate in white matter lesions (both adult and paediatric MS) and in B‐cell infiltration within the meninges and parenchymal B‐cell aggregates in MS brain tissue.14 In another study, EBV‐specific transcripts were not detected in active and chronic active MS plaques rich in perivascular B‐lymphocyte cuffs or in single B lymphocytes and in plasma cells isolated from MS patients' CSF.15 The negative results raised questions about the prevalence of active EBV infection in MS brain.
CD8+ CD28– CD57+ T cells seem to have a central role in the response to EBV and in MS. Defined as an effector/cytotoxic population even more potent than natural killer cells in releasing cytotoxic granules,16 CD8+ CD28– CD57+ T cells increase in EBV infection and arrest the switch to cell memory of EBV‐infected B cells through the release of interferon‐γ (IFN‐γ).17 In vitro studies have shown that EBV infection induces the release of inflammatory cytokines like interleukin‐12 and type I IFNs by the activation of innate immune cells18, 19 and dendritic cells activated by EBV products prime naive T cells to recognize EBV‐infected B cells.20 Interestingly, a high frequency of myelin‐specific CD8+ CD28‐ CD57+ T cells was discovered in MS patients, suggesting that myelin‐reactive CD8+ T cells are chronically stimulated in patients with MS.21 Effector polyfunctional CD8+ CD28‐ CD57+ T cells increased and persisted in the peripheral blood of patients with MS during glatiramer acetate treatment22, 23 and after bone marrow transplantation for treatment of aggressive forms of the disease.24 Although CD8+ CD57+ T cells were observed increasing in the blood of patients with MS after disease‐modified drugs and therapy leading to the reconstitution of the immune system, their role in the disease remains unknown.
An anergic exhaustion‐like phenotype regulated through the programmed death 1/programmed death ligand 1 and 2 (PD‐1/PDL‐1 and PDL‐2) pathway has been described recently in virus‐specific CD8+ T cells in chronic human immunodeficiency virus,25, 26 hepatitis C virus,27, 28 hepatitis B virus28, 29 and human T‐lymphotropic virus infections;30 effector CD8+ T cells can become unresponsive to viral antigens and consequently fail to eliminate the viral load.31, 32 PD‐1 and human inhibitor receptor immunoglobulin‐like transcript 2 (ILT2/CD85j) regulate activation, proliferation and cytokine release from CD8+ T cells and natural killer cells, respectively.33, 34, 35, 36
In this study, we have investigated the role of PD‐1 and ILT2/CD85j in regulating EBV‐specific CD8+ CD57+ T cells and the association of PD‐1 and ILT2 expression with disease activity in MS. To address these issues, we have investigated the expression of the degranulation marker CD107a, the release of pro‐inflammatory cytokines and cytotoxic granules by CD8+ CD57+ T cells, and their ability to kill EBV‐infected cells. We then correlated the activity of CD8+ CD57+ T cells to the expression of the inhibitory receptors PD‐1 and ILT2 in healthy donors and in patients with MS in stable versus active phases of the disease.
We demonstrate that CD8+ CD57+ T cells are polyfunctional effector cells with a strong ability to recognize and to kill EBV‐infected B cells. Moreover, we find that the expression of the PD‐1 receptor modulates the activation and cytokine release by CD8+ CD57+ T cells. We observe significant up‐regulation of the expression of PD‐1, indicating an exhausted phenotype in CD8+ CD57+ T cells in patients with MS in the remitting phase of the disease compared with healthy donors, supporting the hypothesis of impaired responses to EBV in patients with MS. Interestingly, we have detected infiltrates of CD8+ CD57+ T cells in the meninges of post‐mortem brain tissue of patients with MS with rapidly progressive disease suggesting the likely involvement of these cells in the disease process.
Materials and methods
Patients
Blood samples were collected from 36 healthy donors and 35 patients with a diagnosis of relapsing–remitting MS (RR‐MS). The project received ethics committee approval from the National Research Ethics Service (reference number 05/MRE12/8) and patients gave their written informed consent before taking part in the study. Patients with MS were categorized as stable if they were in clinical remission for at least 3 months and did not present gadolinium‐enhancing lesions on brain magnetic resonance imaging, or active if they had presented clinical relapses in the previous 3 months before the enrolment and the blood samples were obtained before any corticosteroid therapy. At the time of blood sampling, all recruited patients were either untreated or during a 2‐month washout between therapies. The patients’ demographic and basic clinical characteristics and their prior treatments are shown in Table 1.
Table 1.
Demographic and clinical characteristics of RRMS patients
| Age | Sex | EDSS | Relapses in the last 2 years | Treatment | DMT before wash out | |
|---|---|---|---|---|---|---|
| Active patients | ||||||
| MS1 | 43 | F | 3 | 3 | WO | Avonex |
| MS2 | 46 | M | 3·5 | 2 | NT | NT |
| MS3 | 50 | F | 3·5 | 3 | WO | Tysabri |
| MS4 | 34 | F | 6 | 3 | WO | Tysabri |
| MS5 | 25 | F | 6 | 2 | WO | Glatiramer Acetate |
| MS6 | 30 | F | 3 | 2 | WO | Rebif |
| MS7 | 31 | F | 4 | 3 | NT | NT |
| MS8 | 36 | F | 4 | 2 | WO | Avonex |
| MS9 | 43 | M | 3 | 2 | NT | NT |
| MS10 | 43 | F | 3·5 | 2 | WO | Avonex |
| MS11 | 40 | F | 4 | 3 | WO | Fingolimod (FTY720) |
| MS12 | 54 | M | 3 | 2 | WO | Avonex |
| MS13 | 46 | F | 3 | 2 | WO | Avonex |
| MS14 | 27 | M | 3·5 | 3 | WO | Tysabri |
| Stable patients | ||||||
| MS15 | 32 | F | 1 | 0 | NT | N/A |
| MS16 | 27 | F | 1 | 0 | NT | N/A |
| MS17 | 30 | M | 1·5 | 0 | NT | N/A |
| MS18 | 47 | M | 1·5 | 0 | NT | N/A |
| MS19 | 32 | F | 1 | 0 | NT | N/A |
| MS20 | 33 | F | 1·5 | 0 | NT | N/A |
| MS21 | 37 | F | 1·5 | 1 | NT | N/A |
| MS22 | 28 | F | 1 | 0 | NT | N/A |
| MS23 | 53 | F | 3 | 1 | NT | N/A |
| MS24 | 25 | F | 1·5 | 0 | NT | N/A |
| MS25 | 29 | F | 1·5 | 0 | NT | N/A |
| MS26 | 55 | M | 2 | 0 | NT | N/A |
| MS27 | 40 | F | 1·5 | 0 | NT | N/A |
| MS28 | 48 | F | 2 | 1 | NT | N/A |
| MS29 | 43 | F | 2 | 0 | NT | N/A |
| MS30 | 32 | F | 1·5 | 0 | NT | N/A |
| MS31 | 34 | F | 1·5 | 0 | NT | N/A |
| MS32 | 38 | F | 2 | 1 | NT | N/A |
| MS33 | 35 | M | 2 | 1 | NT | N/A |
| MS34 | 37 | F | 1·5 | 1 | NT | N/A |
| MS35 | 45 | F | 2 | 0 | NT | N/A |
EDSS, Expanded Disability Status Scale; DMT, disease modified therapy; NT, not treated; WO, wash out; NA, not applicable.
Cells and flow cytometry
Peripheral blood mononuclear cells (PBMC) were isolated from blood samples as described.37The cells were counted by using trypan blue solution (Sigma‐Aldrich Co Ltd, Irvine, UK) and suspended at 1 × 106 cells/ml in RPMI‐1640 complete medium.
Fresh PBMC were stimulated with PMA and ionomycin (Iono) (Sigma‐Aldrich), 500 nm and 50 nm, respectively, for 5 hr at 37° with 5% CO2. Brefeldin A (Sigma‐Aldrich) was added for the last 4 hr at a concentration of 5 μm. Cells were then collected and prepared for surface and intracellular staining as described below.
Surface staining: PBMCs were stained with the following surface antibodies: phycoerythrin (PE) ‐conjugated anti‐CD3 (BD Biosciences, Oxford, UK), allophycocyanin (APC) ‐conjugated anti‐CD3 (BD Biosciences), PE‐Cy7‐conjugated anti‐CD8 (eBioscience, San Diego, CA), FITC‐conjugated anti‐CD57 FITC (Life Technologies Ltd, Paisley, UK), APC‐conjugated anti‐PD‐1 and APC‐conjugated anti‐ILT2 APC (eBioscience) on ice for 15 min; cells were then washed in PBS and sample acquisition and analysis were performed on a FACScalibur flow cytometer (BD UK Limited, Berkshire, UK).
Intracellular staining: after surface staining, the cells were washed in PBS and the pellet was suspended in fixation buffer (BD Biosciences) for 20 min at room temperature in the dark. Then, the cells were washed in permeabilization buffer 1 × (BD Biosciences) and stained with the following intracellular antibodies: APC‐conjugated anti‐Granzyme B, anti‐Perforin and anti‐IFN‐γ; PE‐Cy7‐conjugated anti‐ macrophage inflammatory protein 1β (MIP‐1β; eBioscience) and PE‐conjugated anti‐interleukin 2 (BD Biosciences).
EBV‐containing supernatant: marmoset cell line B95‐8 cells were suspended in complete medium at a concentration of 1 × 106 and left for 3 days in a humidified 37°, 5% CO2 incubator. Then, the supernatant was centrifuged at 270 g, at 4° to separate the EBV‐containing culture supernatant from the cells and filtered through a 0·45‐μm filter.
Autologous EBV‐infected B‐lymphoblastoid cell lines (B‐LCL): 2 × 106 PBMC were centrifuged and the pellet was infected for 2 hr with 2·5 ml of EBV‐containing culture supernatant obtained as described above and left in the incubator. Then, 5 ml of RPMI complete medium containing 1 μg/ml cyclosporine A was added and the cells were left in the incubator until microscopic clumps were evident and the culture medium became acidic. The cells were split weekly with the addition of RPMI complete medium.
Degranulation assay
PBMC were seeded at 1 × 106 cells/well in polystyrene 96‐well V‐bottomed plates coated with purified anti‐CD3 10 μg/ml (OKT3, eBioscience).16 The assay was performed as described previously.38 In blocking experiments, PBMC were stained with purified anti‐PD‐1 antibody (eBioscience) on ice for 15 min. Then, the cells were washed with PBS and stained with purified anti‐IgG antibody (eBioscience) on ice for 15 min, washed again and stimulated in 96‐well V‐bottomed plates as described above. After 4 hr, the cells were collected, washed with PBS and stained for surface and intracellular markers and acquired as described above.
Evaluation of cytotoxicity
P815 murine cell lines and autologous B‐LCL were stained with 5 μm carboxyfluorescein succinimidyl ester (CFSE) as described previously.39 B‐LCL were also stained with anti‐CD19 APC to separate the lymphoblastoid cell line from lymphocytes. Sorted CD8+ CD57+/– T cells (CD8+ CD57+ T kit; Miltenyi Biotec Ltd, Surrey, UK) were seeded with a constant number of target cells (50 000) at different effector to target (E : T) ratios in anti‐CD3 (10 μg/ml, Thermo Fisher Scientific) pre‐coated 96‐well V‐bottomed plates in a total volume of 200 µl and left in the incubator for 4 hr as described elsewhere.16, 40, 41 In parallel, target cells were incubated alone to measure spontaneous apoptosis and used for comparative analysis. Then, 1 μm of 7‐aminoactinomycin B (7‐AAD; Sigma‐Aldrich) was added for 15 min on ice in the dark and the sample was then acquired on the flow cytometer.41 For each E : T ratio, 20 000 target cells were acquired. Dead target cells were detected as double‐positive CFSE‐7‐AAD cells. The percentage of cytotoxic activity (delta cells) was calculated by using the following equation:
Co‐culture of PBMC and autologous B‐LCL
PBMC were seeded at 1 × 106 cells/ml per well or in transwells with or without autologous B‐LCL for 5 days. Then, the cells were collected and seeded on anti‐CD3 coated V‐bottomed plates for 4 hr in the presence of PE‐conjugated anti‐CD107a as described previously.38 Then the cells were collected and stained for surface markers and intracellular cytokines as reported above.
Detection of apoptotic cells
PBMC were seeded and stimulated with anti‐CD3 at concentrations of 1 and 5 µg/ml for 4 and 24 hr. Then the cells were collected, stained for surface receptors, Annexin V (1 μg/ml, eBioscience) and 7‐AAD (1 μg/ml) and acquired on the flow cytometer.
Detection of CD8+ CD57+ T cells in post‐mortem MS tissue
Brains were obtained at autopsy from the UK MS Society Tissue Bank at Imperial College, under ethical approval by the National Research Ethics Committee (08/MRE09/31). Post‐mortem tissue material from individuals with RR‐MS was not available for our study. To make our brain tissue analysis of the expression of CD57 more closely representative of the inflammatory pathology expected in RR‐MS, we selected from the available secondary progressive multiple sclerosis (SP‐MS) cases those who presented on‐going active demyelination characterized by the presence of high levels of meningeal and perivenular inflammatory infiltrates. The presence of CD3+ and CD8+ CD57+ T cells, both in the perivascular spaces and in the meninges, was evaluated by immunofluorescence and immunohistochemistry on 4% paraformaldehyde fixed frozen section or formaldehyde‐fixed paraffin sections, respectively, from 10 post‐mortem SP‐MS patients with rapidly progressive disease (see Supplementary material, Table S1). For each of the examined SP‐MS cases, five perivascular infiltrates and five meningeal infiltrates, randomly selected among different brain regions, were examined and the percentage of CD3+ CD57+ cells out of the total CD3+ T‐cell number was evaluated for each infiltrate. De‐waxed 5‐μm‐thick paraffin sections were microwave‐processed in citrate buffer (pH 6) and immunostained by using mouse anti‐CD3 antibody (Clone PS1; Immunotech, Marseille, France), mouse anti‐CD8 (clone 4B11; Invitrogen, Rockford, IL) or mouse anti‐PD‐1 (eBioscience), followed by signal detection using the avidin‐biotin‐peroxidase method, haematoxylin counterstain and mounting with Canadian Balsam, as previously described.8, 42 Images were acquired and analysed with an Axiophot microscope (Carl Zeiss, Jena, Germany) equipped with a digital camera (Axiocam HRC). For double immunofluorescence, rabbit anti‐CD57 antibody (Abcam, Cambridge, MA) was used with mouse anti‐CD3 or anti‐CD8 or PD‐1 antibodies followed by combination of Alexa‐fluor 488‐conjugated donkey anti‐mouse IgG (Invitrogen, Eugene, OR) with Cy3‐conjugated donkey anti‐rabbit IgG (Jackson Immunoresearch Laboratories, West Grove, PA). Sections were sealed in ProLong Gold antifade reagent with 4′,6′‐diamidino‐2‐phenylindole (DAPI) (Invitrogen) and images were acquired and analysed with epi‐fluorescence microscope Axiophot (Carl Zeiss) equipped with a digital camera (Axiocam HRM).
Statistical analysis
The statistical analyses were performed using graphpad prism software (version 6; GraphPad Software, La Jolla, CA) using the appropriate following tests as specified in the figure legends: Mann–Whitney non‐parametric t‐test, Wilcoxon t‐test, two‐way analysis of variance Sidak's multiple comparison, Welch's non‐parametric t‐test, one‐way analysis of variance Turkey's multiple comparisons test and Pearson correlation coefficient. Statistical significance was retained for P‐values < 0·05.
Results
Pro‐inflammatory and cytotoxic profile of CD8+ CD57+ T cells
CD57+/– CD8+ T cells were investigated for the release of a panel of pro‐inflammatory cytokines and cytotoxic granules as shown in Fig. 1(a). CD8+ CD57+ T cells release mainly T helper type 1 cytokines such as IFN‐γ and MIP‐1β. CD57+ T cells are effector cytotoxic cells rich in cytotoxic granules such as perforin and granzyme B. Moreover, upon T‐cell receptor (TCR) stimulation CD8+ CD57+ T cells were also able to degranulate more efficiently compared with the CD8+ CD57– counterpart as shown by the expression of CD107a16 (Fig. 1b,c), confirming their higher cytotoxic potential.
Figure 1.
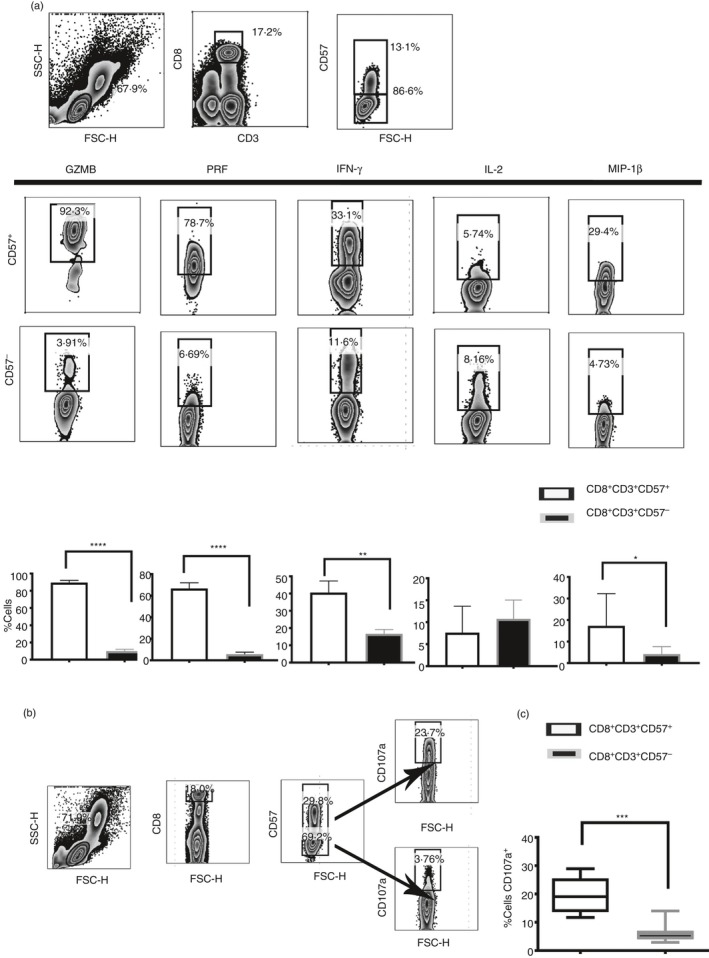
Polyfunctional phenotypes of CD8+ CD57+ T cells. Pro‐inflammatory cytokines and cytotoxic granules were detected by intracellular flow cytometry. The peripheral blood mononculear cells (PBMC) were stimulated with PMA and Ionomycin (Iono) in the presence of Brefeldin A. (a) Release of granzyme B (GZMB), interferon‐γ (IFN‐γ), perforin (Perf), interleukin‐2 (IL‐2) and macrophage inflammatory protein 1β (MIP1β). Dot plots showed the flow cytometry gating strategy that we have used to identify cytokines and cytotoxic profile in CD8+ CD57+/– T cells. (b and c) Cytotoxic phenotype was confirmed by the CD107a expression and compared in CD8+ CD57+/– T cells. Data from eight experiments shown as mean ± SEM, Mann–Whitney non‐parametric t‐test *P < 0·05, **P < 0·01, ***P < 0·001, ****P < 0·0001.
Donor variance of PD‐1 and ILT2/CD85j expression on CD8+ CD57+ T cells
Considering the cytotoxic function of CD8+ CD57+ T cells and their established role in the response to viruses, and given the regulatory role of the receptors PD‐1 and ILT2 in virus‐specific effector cells, we have investigated their expression on the CD8+ CD57+ T‐cell population in a group of 30 healthy donors (see Supplementary material, Fig. S1a). We detected a considerable variance in the individual donors’ expression of ILT2 and PD‐1 on CD8+ CD57+ T cells (Fig. 2a) and demonstrate a linear weak correlation between PD‐1 and ILT2 expression on CD8+ CD57+ T cells (Fig. 2b, *P < 0·05). This result supposes an independent function of the two receptors.
Figure 2.
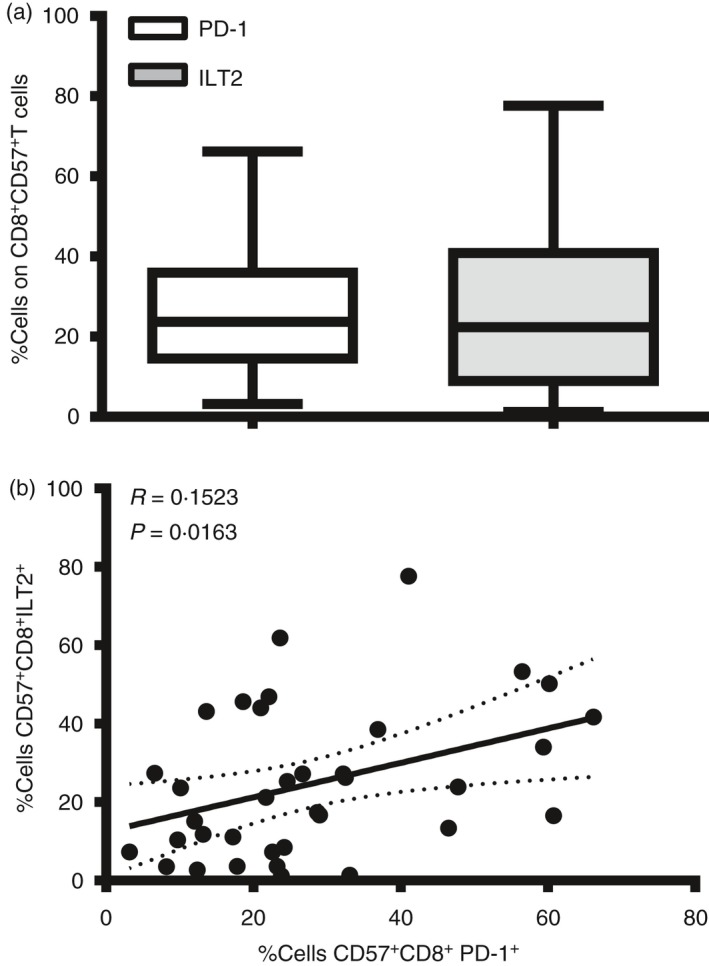
Donor variance of programmed death 1 (PD‐1) and immunoglobulin‐like transcript 2 (ILT2) expression on CD8+ CD57+ T cells. (a) Percentage of cells positive to ILT2 and PD‐1 receptor on CD8+ CD57+ T cells from 30 healthy donors were shown in bar histograms. Data represents Min to Max. (b). Linear correlation between ILT2 and PD‐1 receptors on CD8+ CD57+ T cells from 30 healthy donors.
PD‐1 expression inversely correlates with cytokine release and lytic degranulation on effector cytotoxic CD8+ CD57+ Tcells
Considering that PD‐1 and ILT2/CD85j inhibit T‐cell activation, cytokine production and natural killer IFN‐γ release,36, 43 we have investigated the role of these two inhibitory receptors in the regulation of effector CD8+ CD57+ T‐cell function. As shown in Fig. 3(a), the content of the granules released by CD8+ CD57+ T cells upon TCR triggering was identified by the co‐expression of the lysosomal marker CD107a with granzyme B, perforin and IFN‐γ. We also examined the correlation between granule content with PD‐1 (Fig. 3b) and ILT2 expression (see Supplementary material, Fig. S1b) on CD8+ CD57+ T cells. CD8lo CD57+ cells also were excluded in the analysis since they are natural killer cells as shown in the Supplementary material (Fig. S2). There was an inverse correlation between CD107a and PD‐1 expression on CD8+ CD57+ T cells (**P < 0·01, *P < 0·05, respectively). Hence, the increased expression of PD‐1 on CD8+ CD57+ T cells correlates with the progressive loss of the ability to release IFN‐γ, perforin and granzyme B (*P < 0·05, **P < 0·01). No significant correlation with ILT2 was observed.
Figure 3.
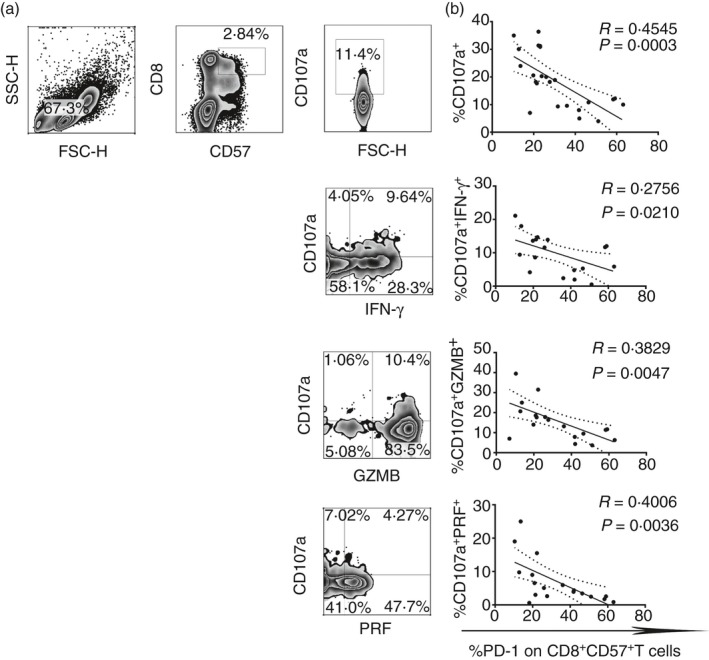
Programmed death 1 (PD‐1) expression inversely correlates with cytokine release and lytic degranulation on effector cytotoxic CD8+ CD57+ T cells. (a) Dot plots show the flow cytometry gating strategy that we used to define the percentage of cells CD107a+, CD107a+ IFN=γ +, CD107a+ PERF + and CD107a+ GZMB + on CD8+ CD57+ T cells upon T‐cell receptor stimulation. (b) Linear correlation between degranulation of granzyme B (GZMB), perforin (PERF), interferon‐γ (IFN‐γ) and PD‐1 expression on CD8+ CD57+ T cells from 19 healthy donors.
CD8+ CD57+ T cells increase CD107a expression and cytotoxicity in response to autologous EBV‐infected lymphoblastoid B cell lines (B‐LCL)
We performed co‐culture experiments with autologous EBV B‐LCL and investigated the reactivity of CD8+ CD57+ T cells to EBV compared with the CD8+ CD57– counterpart by evaluating degranulation and release of cytokines. CD8+ CD57+ T cells showed a significant increase in the expression of CD107a (*P < 0·05), in granzyme B degranulation and in IFN‐γ release (*P < 0·05) when PBMC were incubated with autologous EBV B‐LCL or in transwells compared with PBMC alone (Fig. 4). No significant activation was observed on CD8+ CD57– T cells. These results demonstrate that CD57 is a marker expressed by EBV‐specific effector and cytotoxic CD8+ T cells.
Figure 4.
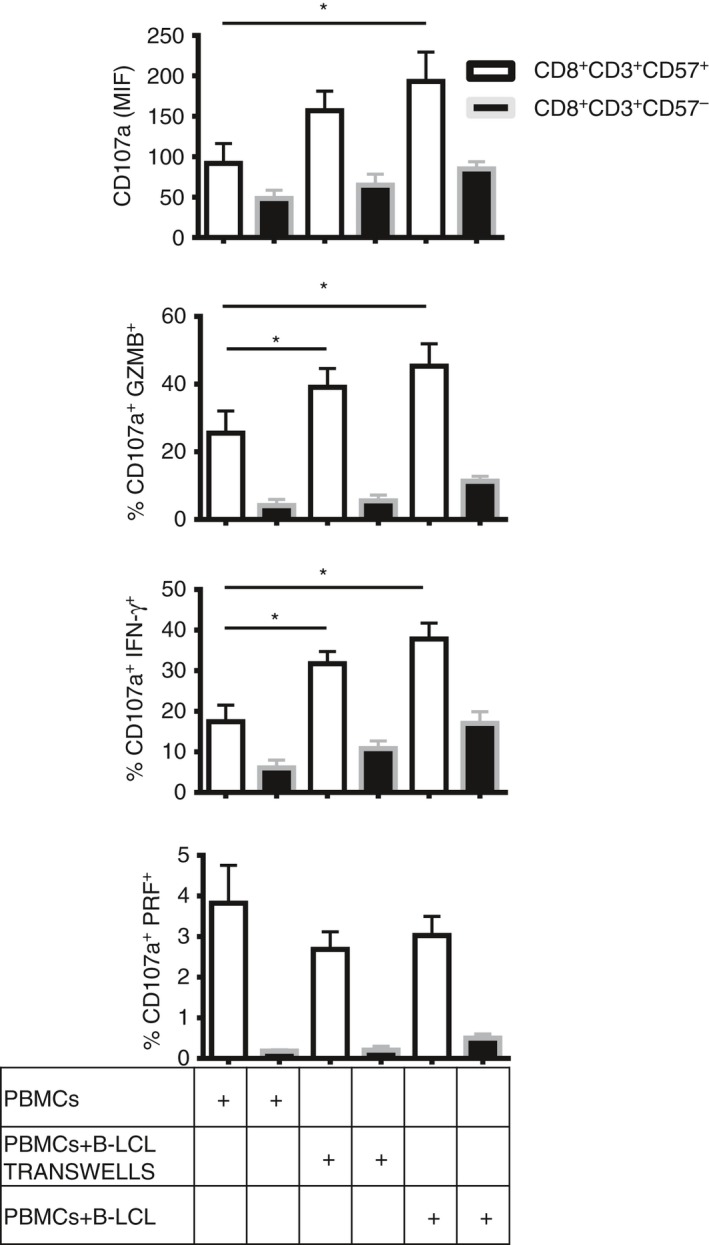
CD8+ CD57+ T cells increase CD107a expression and cytotoxicity in response to autologous Epstein–Barr virus (EBV)‐infected lymphoblastoid B cell lines (B‐LCL). Peripheral blood mononculear cells (PBMC) from six healthy donors were incubated alone, with autologous EBV B‐LCL or in transwells for 5 days. Then, the cells were collected and stimulated with anti‐CD3 for 4 hr in the presence of anti‐CD107a, brefeldin A and monensin. Cytokine release and degranulation were investigated. Mean intensity fluorescence (MIF) of CD107a, percentage of cells CD107a+ IFN‐γ +, CD107a+ GZMB + and CD107a+ PRF + on CD8+ CD57+/– T cells were shown as bar histograms. Data from six experiments are shown as mean ± SEM, Mann–Whitney non parametric t‐test, *P < 0·05.
PD‐1 expression on CD8+ CD57+ T cells is associated with reduced cytotoxicity to the p815 murine cell line and autologous EBV B‐LCL
CD8+ CD57+ T cells from different donors showed varying degrees of cytotoxicity to p815 and autologous B‐LCL. We observed an inverse correlation between cell death and PD‐1 expression at E : T cell ratios of 0·5 : 1 and 1 : 1 in p815 cell line experiments and at a ratio of 30 : 1 in EBV‐infected B‐LCL experiments, respectively (*P < 0·05) (Fig. 5c,d). The results suggest that PD‐1 expression is negatively associated with cytotoxicity of CD57+ CD8+ T cells. Furthermore, the expression by autologous EBV B‐LCL of a known ligand for PD‐1, PDL‐1, was investigated. PDL‐1 was indeed expressed by EBV‐infected B‐LCL (see Supplementary material, Fig. S1c). Also, the p815 murine cell line showed expression of PDL‐1 and PDL‐2, as reported.44
Figure 5.
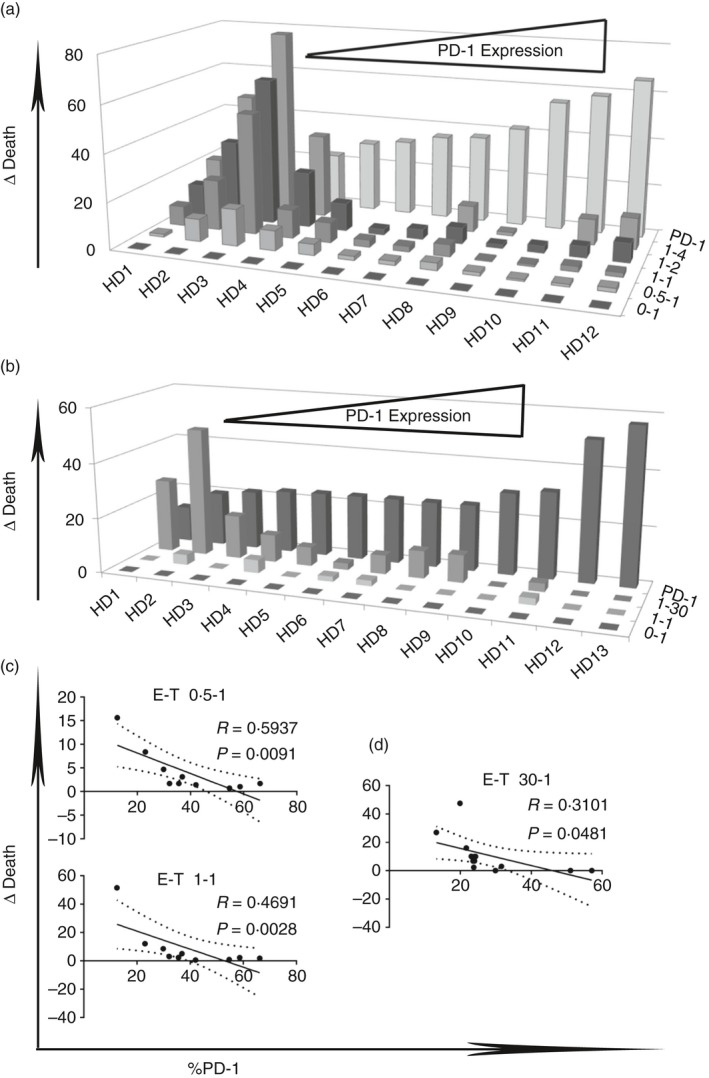
Programmed death 1 (PD‐1) expression on CD8+ CD57+ T cells is associated with reduced cytotoxicity to p815 murine cell line and to autologous Epstein–Barr virus (EBV) ‐infected B‐lymphoblastoid cell lines (B‐LCL). D Death of p815 cell lines (a) and autologous EBV‐infected B‐LCL (b) were correlated to PD‐1 expression and to effector : target cell ratio (E‐T). Linear correlation between delta of death of p815 cell lines (c) and of autologous EBV B‐LCL (d) and PD‐1 expression at several effector : target cell ratios.
Blockade of PD‐1 receptor restores cytotoxicity to target cells and cytokine release in CD8+ CD57+ T cells
Having detected a correlation between PD‐1 expression and the reduced cytotoxicity against autologous B‐LCL, in subsequent experiments we hypothesized that the degranulation of cytotoxic granules, the cytokine release by CD8+ CD57+ T cells and cytotoxicity to autologous EBV‐infected B‐LCL could be enhanced following blocking of PD‐1. Indeed, blocking PD‐1 significantly increased the degranulation of granzyme B and perforin and IFN‐γ release compared with untreated controls (Fig. 6a,b, *P < 0·05).
Figure 6.
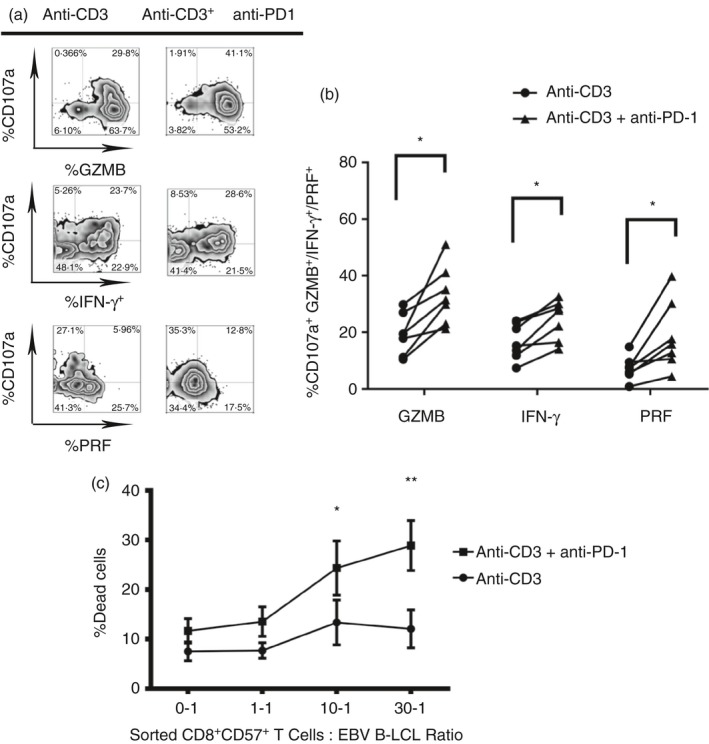
Blockade of programmed death 1 (PD‐1) receptor restores cytotoxicity to target cells and cytokine release on CD8+ CD57+ T cells. Blockade of PD‐1 is performed on experiments of cytokine release and degranulation upon T‐cell receptor (TCR) stimulation. Peripheral blood mononculear cells (PBMCs) from healthy donors were pre‐treated with anti‐PD‐1 for 30 min in ice. The cells were washed and treated with anti‐IgG for 30 min in ice. After, they were seeded in anti‐CD3 coated 96‐well V‐bottomed plates and left in an incubator for 4 hr in the presence of CD107a, brefeldin A and monensin. Then, the cells were stained for surface receptors and intracellular cytokines and cytotoxic granules and the samples were acquired and analysed. (a) Dot plots show gating strategy used to define cytokine release and degranulation of cytotoxic granules. (b) Data from six experiments are shown, *P < 0·05, **P < 0·01, Wilcoxon t‐test. (c) Blockade of PD‐1 was also performed in cytotoxicity to autologous Epstein–Barr virus (EBV)‐infected lymphoblastoid B cell lines (B‐LCL). Sorted CD8+ CD57+ T cells were incubated with autologous B‐LCL in anti‐CD3 coated 96V plates to the ratio 0‐1, 1‐1, 10‐1, 30‐1 for 4 hr. The cell death was detected by positivity to 7‐AAD. Data from three experiments are shown. Two‐way analysis of variance Sidak's multiple comparison *P < 0·05, **P < 0·001.
To confirm the functional effect of PD‐1 blocking, sorted CD8+ CD57+ T cells were treated with anti‐PD‐1 and cytotoxicity was measured as the percentage of cell death at increasing E : T cell ratios (Fig. 6c). Blocking of PD‐1 significantly increased the cytotoxicity to EBV B‐LCL at the higher E : T cell ratios (10 : 1 *P < 0·05; 30 : 1 **P < 0·01) compared with cytotoxicity without PD‐1 blocking.
PD‐1 is differentially expressed in healthy donors and patients with MS
We investigated the expression of PD‐1 by CD8+ CD57+ T cells in patients with MS in both the active and stable phase of the disease, not undergoing treatment. Significantly increased expression of PD‐1 was observed on CD8+ CD57+ T cells in patients with stable MS compared with healthy controls (**P < 0·01, Fig. 7a). The expression of PD‐1 in patients with MS with active disease was not significantly different from that of healthy controls.
Figure 7.
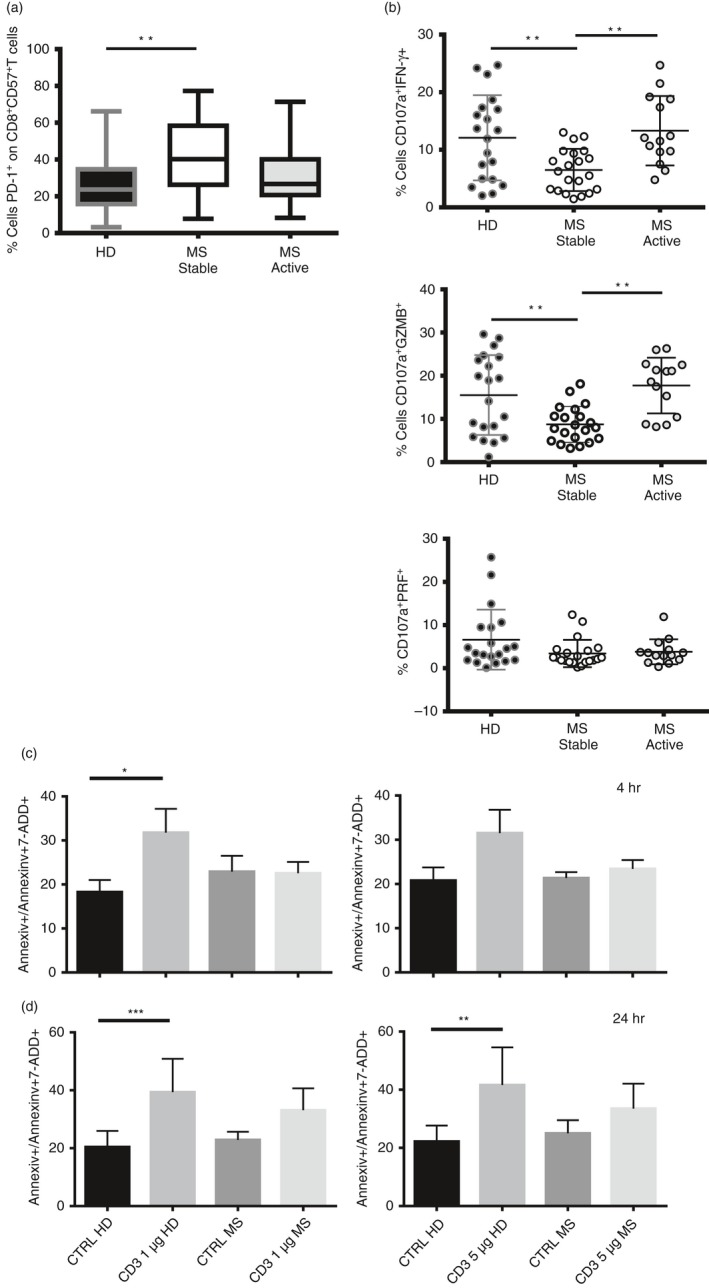
Programmed death 1 (PD‐1) is differentially expressed in healthy donors and patients with multiple sclerosis (MS). (a) Percentage of expression of PD‐1 on CD8+ CD57+ T cells in healthy donors and patients with MS in stable and active disease. Data are from 36 healthy donors, 21 patients with MS in stable disease and 14 patients with MS in active disease. Data shown as Min to Max. Unpaired t‐test with Welch's t‐test, *P < 0·05, **P < 0·01. (b) Percentage of CD107a+ IFN‐γ +, CD107a+ GZMB + and CD107a+ PRF + are shown on CD8+ CD57+ T cells upon T‐cell receptor stimulation in healthy donors and patients with MS in stable and active disease. Data are from 22 healthy donors, 21 patients with remitting MS and 14 patients with relapsing MS and shown as mean ± SD. Ordinary one‐way analysis of variance, Turkey's multiple comparisons test *P < 0·05, **P < 0·01. (c and d) Apoptotic cells (AnnexinV+/AnnexinV+7AAD +) were investigated on CD8+ CD57+ T cells at 4 hr (c) and 24 hr (d) upon anti‐CD3 activation (1 and 5 μg) in healthy donors and patients with MS. Data from four experiments are shown as mean ± SEM, ratio paired t test, *P < 0·05, **P < 0·01.
We then examined the cytokine release and cytotoxic degranulation by CD8+ CD57+ T cells upon TCR activation in the same set of patients with MS. Interestingly, CD8+ CD57+ T cells showed lower release of IFN‐γ and granzyme B in patients with stable MS compared with healthy donors and patients with relapsing disease (Fig. 7b, **P < 0·01). The difference in cytokine release and in degranulation between patients with relapsing MS and active disease was not statistically significant. No significant differences in perforin release were observed among the healthy donors, and in patients with stable MS and active MS.
The fraction of apoptotic cells undergoing activation‐induced cell death was also investigated at different time‐points, namely 4 and 24 hr (Fig. 7c,d, respectively) from TCR activation (1 and 5 μg of anti‐CD3). We observed significant induction of apoptosis in CD8+ CD57+ T cells from healthy controls at 4 hr. No significant apoptosis was observed in patients with MS patients at either 4 or 24 hr. Our results confirm that CD8+ CD57+ T cells from patients with MS show more resistance to activation‐induced cell death than those from healthy donors (*P < 0·05, **P < 0·01, ***P < 0·001).
CD3+ CD57+ T cells are present in inflammatory perivascular and meningeal infiltrates of post‐mortem SP‐MS brain tissue
To verify the presence of CD3+ CD57+ and CD8+ CD57+ cells in MS brains, double immunofluorescence was used on post‐mortem samples from individuals with SP‐MS with rapidly progressive disease characterized by on‐going inflammatory activity at the time of death. The number of CD3+ CD57+ cells found in each infiltrate (Fig. 8a–c), both perivascular and meningeal, corresponded approximately to the number of CD8+ CD57+ cells in the same infiltrates (Fig. 8d–f; see Supplementary material, Table S1). However, occasional CD8– CD57+ cells were also observed in the examined infiltrates (green arrow in Fig. 8f). The percentage of CD57+ cells within the CD3+ T‐cell population in the infiltrates was 10·8% (range 6·6–14%) in the perivascular infiltrates, and 11·8% (range 8–15·4%) in the meninges. PD‐1 expression was also assessed using immunohistochemistry on serial sections from the same post‐mortem MS cases: several scattered PD‐1+ cells were detected, in particular in meningeal infiltrates (Fig. 8g,h). Double immunofluorescence demonstrated that a substantial proportion (20–40%) of the PD‐1+ cells infiltrating the meninges were CD57+ PD‐1+ cells (Fig. 8 insert i in h). Considering the higher frequency of PD1+ cells detected in the meningeal infiltrates compared with the lower number of CD57+ cells, numerous PD‐1+ CD57– cells have been detected in all the examined post‐mortem SP‐MS cases. However, most of the detected CD57+ cells express PD‐1 on their surface.
Figure 8.
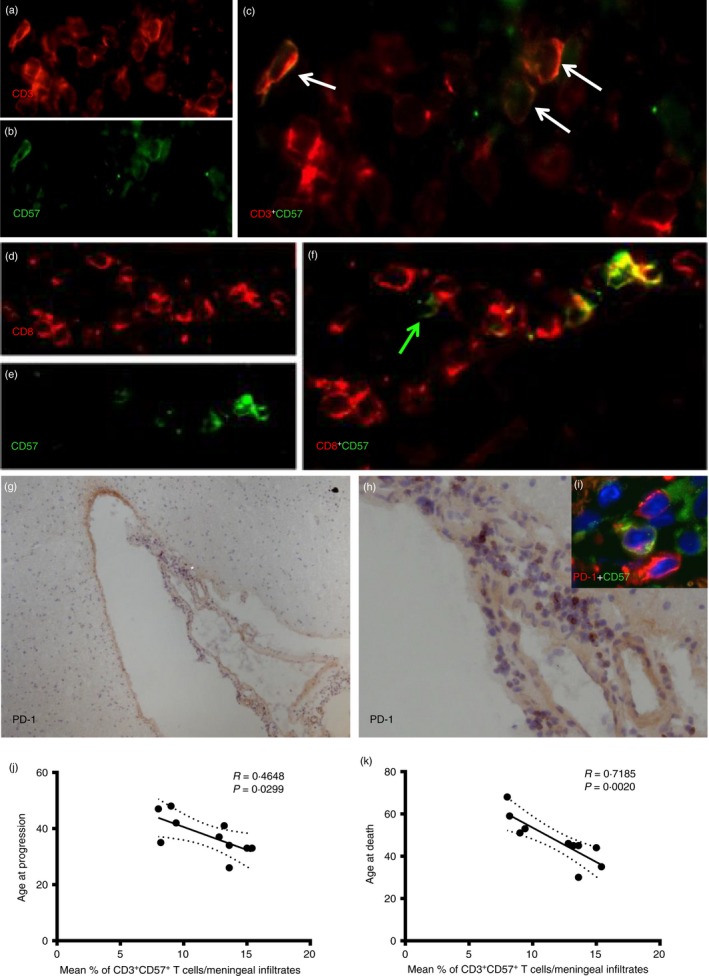
CD3+ CD57+ T cells are present in inflamed perivascular and meningeal infiltrates of post‐mortem secondary progressive multiple sclerosis (SP‐MS) brain tissue. Double immunofluorescence staining was performed on post‐mortem SP‐MS brains. CD3+ CD57+ cells (white arrows) were detected in meningeal infiltrates containing substantial numbers of CD3+ cells (a–c). Also, similar numbers of CD8+ CD57+ cells were detected in the same infiltrates (d–f). However, occasional CD8‐ CD57+ cells were also observed (green arrow, f). By using immunohistochemistry assessment of programmed death 1 (PD‐1) expression on serial sections from the same post‐mortem MS cases, several scattered PD‐1+ cells have been detected in particular in meningeal infiltrates (g and h, higher magnification of a selected area *). Double immunofluorescence demonstrated that a substantial proportion of the PD‐1+ cells infiltrating the meninges are CD57+ PD1+ cells (inset i in h). Negative correlation was found in the meningeal infiltrates between % of CD3+ CD57+ cells and age at disease progression (k) (r = 0·4648, P = 0·0299) and age at death (j) (r = 0·7185; P = 0·0020).
By analysing the possible associations between the proportion of CD57+ cells among all CD3+ cells in immune infiltrates of post‐mortem SP‐MS brains and the clinical details, negative correlations were found in the meningeal infiltrates between % of CD3+ CD57+ cells and age at disease progression (r = 0·4648, P = 0·0299); and age at death (r = 0·7185; P = 0·0020) indicating an association of the relative frequency of CD57+ T cells with the rapidity of disease progression (Fig. 8k,j).
Discussion
In this study, we sought to elucidate the mechanism of effector responses by CD8+ CD57+ T cells against EBV in patients with RR‐MS and to correlate the responses to disease activity status. We demonstrate that CD8+ CD57+ T cells are a population of effector cytotoxic cells with strong ability to kill EBV‐infected cells. A potential role for EBV in MS is supported by strong epidemiological evidence45, 46, 47 but the presence of EBV infection in MS tissue remains controversial.9, 15, 48 A characterization of the cytotoxic CD8+ T‐cell response against EBV, its regulation and its relationship to MS disease activity may contribute to a better understanding of the immunopathological processes in MS and help to develop new therapeutic approaches. Our results show that PD‐1 expression by CD8+ CD57+ T cells is associated with a reduction of degranulation, cytokine release and cytotoxicity towards EBV‐infected targets. This is confirmed by the inverse correlation between functional cytotoxic capacity and PD‐1 expression. We also confirm that blockade of PD‐1 restores CD8+ CD57+ T effector function, specifically allowing more efficient degranulation, cytokine release and cytotoxicity. Detection of a higher frequency of PD‐1 expressing CD8+ CD57+ cells in the blood of patients with RR‐MS during clinical remission, and concomitantly suppressed degranulation, suggests a regulatory role of the molecule on cytotoxic function.
Notably, CD8+ CD57+ T cells were detected in the inflamed meninges and perivascular infiltrates of post‐mortem brains of patients with rapidly progressive MS. Although the tissues were from SP‐MS and not RR‐MS cases, since the latter were not available, selection of cases with high levels of meningeal and perivenular inflammatory infiltrates enabled us to examine inflammatory active pathological processes in post‐mortem brain tissue that is expected to share some similarities with RR‐MS. Previous studies have shown an association between the extent of meningeal infiltration (as well as increased cortical pathology) and a more rapid disease progression.49 On the back of this, our finding of a substantial proportion of CD57+ cells expressing PD‐1 in the meningeal infiltrates of post‐mortem MS cases suggests the involvement of these cells and molecule in the inflammatory immune response. It is plausible to suggest that the inability of CD8+ CD57+ T cells to clear EBV infection in the CNS enables the persistence of chronic inflammation that underlies the observed more rapid progression of MS. However, we cannot rule out that the CD8+ CD57+ T cells’ involvement in the disease process, documented by their detection in MS brain tissue, could be secondary to other inflammatory events occurring in the MS process and that the cells may play a disease‐countering role, potentially through immune regulatory mechanisms, as we suggested in one previous report.50
CD8+ CD57+ T cells are defined as senescent because of short telomeres, low telomerase activity and low cell cycle‐associated genes compared with their CD57 negative counterparts.51 Although senescent, CD8+ CD57+ T cells show a phenotype of terminally differentiated antigen‐specific CD8+ T cells and exhibit lytic granules containing granzyme B and perforin and a great potential cytotoxicity upon TCR stimulation or in co‐culture with autologous EBV‐infected cells compared with CD8+ CD57– T cells, confirming that they are actively responsive to inflammation and pathogens. Moreover, this population expresses high levels of several adhesion molecules (integrin β, α L and β 2, CD11a, ICAM‐I) and reduced levels of CD62L (L‐selectin), showing an ability to migrate to peripheral tissues.52 In addition, CD8+ CD57+ T cells express CX3CR1, the receptor for fractalkine that has been detected in the CSF of patients with early MS and in inflamed MS brain lesions.53, 54 Interestingly, in our study we show that CD8+ CD57+ T cells express high levels of PD‐1 with an exhaustion‐like phenotype in patients with MS in the stable phase of the disease, displaying a weak response upon TCR activation with low release of cytokines and granzyme B. Additionally, CD8+ CD57+ T cells from patients with MS in the active phase of the disease display a low expression of PD‐1, and high release of IFN‐γ and granzyme B, consistent with a profile of terminally differentiated effector/cytotoxic virus‐specific CD8+ T cells. Our results suggest that the inability of cytotoxic T cells to control EBV replication during inactive MS could set the stage for viral reactivation in the CNS and for disease progression.
The fact that anti‐EBV immunity contributes to MS grey matter pathology is strongly supported by an increasing number of studies that correlate cellular and humoral responses to EBV and cortical atrophy or disease activity. CD8+ T‐cell responses to EBV lytic and latent antigens increase in active and inactive MS, respectively, displaying a dysfunctional control of the virus. One longitudinal study described an increase of EBV lytic specific CD8+ T cells, associated with the virus reactivation, during the remission of the disease that anticipates the activity (relapse) of the disease in the central nervous system.11 Also, a cross‐sectional study detected EBV RNA and DNA using quantitative PCR in the peripheral blood of patients with MS and healthy donors.55 It was observed that EBV DNA increases before and during clinical relapse in paired samples suggestive of reactivation of EBV preceding the activity (relapse) of disease in the CNS. In a longitudinal study of patients with RR‐MS without treatment, the expansion of CD8+ T cells specific for EBV lytic antigens has been shown to correlate with the occurrence of relapses and disease activity.56 Impaired CD8 T‐cell responses to EBV in MS, assessed by the frequency of PBMC producing IFN‐γ in response to autologous B‐LCL, were also described.56 Regarding the humoral response to EBV in MS, a significant association between cortical atrophy and anti‐EBV viral capsid antigen and EBNA‐1 antibody status was found in RR‐MS patients.57 In addition, in a previous study on 193 patients with clinically isolated syndrome the highest quartile antibody status of anti‐EBV viral capsid antigen was associated with a greater decrease in thalamus volume and with a trend for a decrease in cortical volume.58 Moreover, a study on serum of pwMS has shown that EBNA‐1 IgG positively correlates with gadolinium‐enhancing magnetic resonance imaging lesions, lesion size and expanded Disability Status Scale in patients with MS59 and in patients with a clinically isolated syndrome with a definitive diagnosis of MS.60 All this evidence supports a less efficient immune control of EBV infection in patients with MS. An impaired immune response to the virus is evident when the infection becomes chronic.27 In this case, the virus persists and effector CD8+ T cells become unresponsive and anergic. In contrast to chronic infection, in acute viral infection, the immune system is highly functional and enables clearance of the virus through cytotoxicity against infected cells and secretion of antiviral factors (e.g. interferon).61 Although those studies support the hypothesis that altered immune reactivity to EBV may favour the reactivation of the virus in intrathecal B cells, which could represent one mechanism causing brain pathology in MS, several studies using similar technologies were unable to detect EBV in the brain tissue and CSF.14, 15, 48, 62 The discrepant results have been the matter of discussion and it has been suggested that methodological differences, including in the preparation and preservation of the brain tissue, may have affected the sensitivity and specificity of EBV detection.63
In our study, we have observed a so‐called exhausted phenotype of CD8+ CD57+ T cells in inactive disease that leads to an inability of these cells to control EBV replication and consequently favours a reactivation of virus. PD‐1 has been described as a marker of activation and differentiation, increasing in early activated EBV‐specific cells and decreasing in terminally differentiated effector cells.64 Low expression of PD‐1 on EBV‐specific effector cytotoxic CD8+ cells enables the mounting of a response to pathogens and clearance of the infection. In patients with MS during remission, the high expression of PD‐1 in CD8+ CD57+ T cells suggests a dysfunctional, reduced cytotoxic response. It is plausible to speculate that PD‐1‐related suppression of cytotoxicity allows MS‐associated viruses such as EBV to persist in the CNS and favours persistent inflammation with tissue damage and consequent relapses. Newly activated CD8+ CD57+ T cells might be induced to clear virus‐infected targets in active MS but the repeated antigenic stimulation and inflammatory cytokines contribute to up‐regulation of PD‐1 with a decrease of effector function.65 Also, resistance to apoptosis induced by activation in CD8+ CD57+ T cells in patients with MS could correlate with dysfunctional expression of inhibitory receptor PD‐166 on these cells and could play a role in dictating the occurrence of inflammatory exacerbations in MS (Fig. 9). One limitation of our study is that we did not examine the potential influence of PD‐1 level on the cytotoxicity against other common viruses such as, for example cytomegalovirus and influenza virus. To study EBV responses in the most physiologically relevant way, we generated autologous EBV‐LCL to be used as target in the functional assays, focused on the modulation of cytotoxic function by PD‐1. As EBV is unique in its ability to induce stable lymphoblastoid cell lines, such an approach could not be used for cytomegalovirus67, 68 or any non‐lymphotropic viruses such as influenza virus. In the absence of a comparison we cannot rule out that the dysfunction of cytotoxic activity against EBV demonstrated by our results in patients with MS might also be observed against other viruses.
Figure 9.
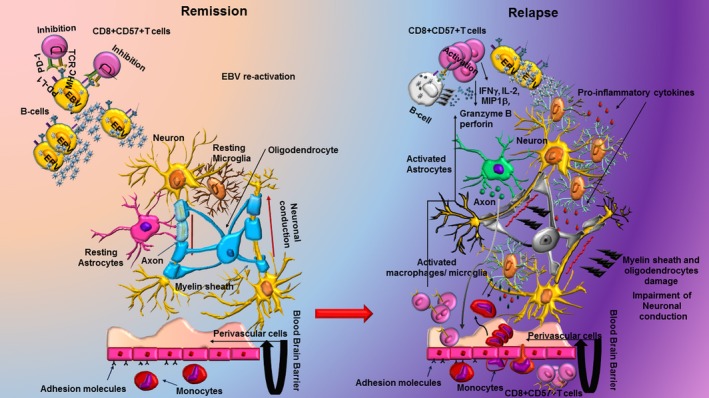
Programmed death 1 (PD‐1) on CD8+ CD57+ regulates the response to Epstein–Barr virus (EBV) and the reactivation of the virus in the central nervous system (CNS) of patients with multiple sclerosis (MS). In patients with MS during remission, the high expression of PD‐1 in CD8+ CD57+ T cells suggests a dysfunctional, reduced cytotoxic response that allows MS‐associated viruses such as EBV to persist in the CNS and favours persistent inflammation with tissue damage and consequent relapses. Newly activated CD8+ CD57+ T cells might be induced to clear virus‐infected targets in active MS but the repeated antigenic stimulation and inflammatory cytokines contribute to up‐regulation of PD‐1 with a decrease of effector function.
Taken together, our data demonstrate that CD8+ CD57+ T cells are polyfunctional effectors that mount cytotoxic and inflammatory responses against EBV‐infected cells and infiltrate MS lesion tissue in substantial numbers correlating with disease severity. Their effector functions are down‐regulated by PD1, which is over‐expressed in stable MS. Our results provide a basis for further studies to evaluate the implication and consider the therapeutic modulation of PD‐1 expression in MS.
Author contributions
MTC designed the study, performed experiments, analysed/interpreted data and wrote the manuscript. RM performed immunostaining experiments on MS brain tissue, discussed/analysed data and wrote the manuscript. GB, OM and AR critically revised the manuscripts. RR and RN provided tissue and reagents for immunostaining experiments, and critically revised the manuscript. PAM and LB obtained funding, supervised the study, discussed/interpreted data and critically revised the manuscript.
Disclosures
PM declares honoraria for speaking and travel support from Bayer, Biogen, Merck Serono and Novartis. RN declares compensation and support from Biogen (principal investigator, funds for staff, research, organizing education, honorarium for speaking, advisory boards), Genzyme (honorarium for speaking, advisory boards, organizing education), NICE diagnostics advisory committee, Expert NICE Alemtuzumab committee; Novartis (principal investigator, honorarium for speaking, advisory boards), Roche (advisory boards). None of the other co‐authors has a financial or other conflict of interest.
Supporting information
Figure S1. (a) Dot plots show the gating strategy used to define immunoglobulin‐like transcript 2 (ILT2) and programmed death‐1 (PD‐1) expression on CD8+ CD57+ T cells. (b) Linear correlation between degranulation of granzyme B (GZMB), perforin (PERF) and interferon‐γ (IFN‐γ) and ILT2 expression on CD8+ CD57+ T cells from 19 healthy donors, Pearson *P < 0·05, **P < 0·01). (c) Dot plot shows the PDL‐1 expression on autologous Epstein–Barr virus (EBV)‐infected lymphoblastoid B cell lines (B‐LCL)
Figure S2. CD8high/low CD57+ are investigated for expression of CD3.
Table S1. Demographic, clinical, autopsy characteristics of the multiple sclerosis cases analysed.
Acknowledgements
This study was supported by a research fellowship to MTC from Fondazione Italiana Sclerosi Multipla (FISM; ref. 2013/B/2). The study was also supported by grants awarded from FISM ref. 2015/R/16 to PM, FISM ref. 2013/R/2 and Italian Ministry of Health, Italy RF‐2011‐02347228 to L.B. We are grateful to all participants who donated blood for this study. We thank Caroline D'Arcy for research nursing support and Dr Esther Morel for technical support. Brain post‐mortem tissue samples and the associated clinical and neuropathological data were supplied by the Multiple Sclerosis Society Tissue Bank, funded by the UK Multiple Sclerosis Society.
References
- 1. Compston A, Coles A. Multiple sclerosis. Lancet 2008; 372:1502–17. [DOI] [PubMed] [Google Scholar]
- 2. Salvetti M, Giovannoni G, Aloisi F. Epstein–Barr virus and multiple sclerosis. Curr Opin Neurol 2009; 22:201–6. [DOI] [PubMed] [Google Scholar]
- 3. Mechelli R, Manzari C, Policano C, Annese A, Picardi E, Umeton R et al Epstein–Barr virus genetic variants are associated with multiple sclerosis. Neurology 2015; 84:1362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cepok S, Zhou D, Srivastava R, Nessler S, Stei S, Bussow K et al Identification of Epstein–Barr virus proteins as putative targets of the immune response in multiple sclerosis. J Clin Invest 2005; 115:1352–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lunemann JD, Edwards N, Muraro PA, Hayashi S, Cohen JI, Munz C et al Increased frequency and broadened specificity of latent EBV nuclear antigen‐1‐specific T cells in multiple sclerosis. Brain 2006; 129:1493–506. [DOI] [PubMed] [Google Scholar]
- 6. Jilek S, Schluep M, Meylan P, Vingerhoets F, Guignard L, Monney A et al Strong EBV‐specific CD8+ T‐cell response in patients with early multiple sclerosis. Brain 2008; 131:1712–21. [DOI] [PubMed] [Google Scholar]
- 7. Serafini B, Rosicarelli B, Franciotta D, Magliozzi R, Reynolds R, Cinque P et al Dysregulated Epstein–Barr virus infection in the multiple sclerosis brain. J Exp Med 2007; 204:2899–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Magliozzi R, Serafini B, Rosicarelli B, Chiappetta G, Veroni C, Reynolds R et al B‐cell enrichment and Epstein–Barr virus infection in inflammatory cortical lesions in secondary progressive multiple sclerosis. J Neuropathol Exp Neurol 2013; 72:29–41. [DOI] [PubMed] [Google Scholar]
- 9. Serafini B, Muzio L, Rosicarelli B, Aloisi F. Radioactive in situ hybridization for Epstein–Barr virus‐encoded small RNA supports presence of Epstein–Barr virus in the multiple sclerosis brain. Brain 2013; 136 (pt7):e233. [DOI] [PubMed] [Google Scholar]
- 10. Tzartos JS, Khan G, Vossenkamper A, Cruz‐Sadaba M, Lonardi S, Sefia E et al Association of innate immune activation with latent Epstein–Barr virus in active MS lesions. Neurology 2012; 78:15–23. [DOI] [PubMed] [Google Scholar]
- 11. Angelini DF, Serafini B, Piras E, Severa M, Coccia EM, Rosicarelli B et al Increased CD8+ T cell response to Epstein–Barr virus lytic antigens in the active phase of multiple sclerosis. PLoS Pathog 2013; 9(4):e1003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Nierop GP, Janssen M, Mitterreiter JG, van de Vijver DAMC, de Swart RL, Haagmans BL et al Intrathecal CD4+ and CD8+ T‐cell responses to endogenously synthesized candidate disease‐associated human autoantigens in multiple sclerosis patients. Eur J Immunol 2016; 46:347–53. [DOI] [PubMed] [Google Scholar]
- 13. van Nierop GPMJ, Mitterreiter JG, Hintzen RQ, Verjans GM. Intrathecal CD8 T‐cells of multiple sclerosis patients recognize lytic Epstein–Barr virus proteins. Mult Scler 2016; 22:279–91. [DOI] [PubMed] [Google Scholar]
- 14. Willis SN, Stadelmann C, Rodig SJ, Caron T, Gattenloehner S, Mallozzi SS et al Epstein–Barr virus infection is not a characteristic feature of multiple sclerosis brain. Brain 2009; 132:3318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sargsyan SA, Shearer AJ, Ritchie AM, Burgoon MP, Anderson S, Hemmer B et al Absence of Epstein–Barr virus in the brain and CSF of patients with multiple sclerosis. Neurology 2010; 74:1127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chiang SCC, Theorell J, Entesarian M, Meeths M, Mastafa M, Al‐Herz W et al Comparison of primary human cytotoxic T‐cell and natural killer cell responses reveal similar molecular requirements for lytic granule exocytosis but differences in cytokine production. Blood 2013; 121:1345–56. [DOI] [PubMed] [Google Scholar]
- 17. Sohlberg E, Saghafian‐Hedengren S, Rasul E, Marchini G, Nilsson C, Klein E et al Cytomegalovirus‐seropositive children show inhibition of in vitro EBV infection that is associated with CD8+CD57+ T cell enrichment and IFN‐γ. J Immunol 2013; 191:5669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fiola S, Gosselin D, Takada K, Gosselin J. TLR9 contributes to the recognition of EBV by primary monocytes and plasmacytoid dendritic cells. J Immunol 2010; 185:3620–31. [DOI] [PubMed] [Google Scholar]
- 19. Strowig T, Brilot F, Arrey F, Bougras G, Thomas D, Muller WA et al Tonsilar NK cells restrict B cell transformation by the Epstein–Barr virus via IFN‐γ . PLoS Pathog 2008; 4:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bickham K, Goodman K, Paludan C, Nikiforow S, Tsang ML, Steinman RM et al Dendritic cells initiate immune control of Epstein–Barr virus transformation of B lymphocytes in vitro . J Exp Med 2003; 198:1653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ratts RB, Karandikar NJ, Hussain RZ, Choy J, Northrop SC, Lovett‐Racke AE et al Phenotypic characterization of autoreactive T cells in multiple sclerosis. J Neuroimmunol 2006; 178:100–10. [DOI] [PubMed] [Google Scholar]
- 22. Ratts RB, Lovett‐Racke AE, Choy J, Northrop SC, Hussain RZ, Karandikar NJ et al CD28–CD57+ T cells predominate in CD8 responses to glatiramer acetate. J Neuroimmunol 2006; 178:117–29. [DOI] [PubMed] [Google Scholar]
- 23. Karandikar NJ, Crawford MP, Yan X, Ratts RB, Brenchley JM, Ambrozak DR et al Glatiramer acetate (Copaxone) therapy induces CD8+ T cell responses in patients with multiple sclerosis. J Clin Invest 2002; 109:641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muraro PA, Douek DC, Packer A, Chung K, Guenaga FJ, Cassiani‐Ingoni R et al Thymic output generates a new and diverse TCR repertoire after autologous stem cell transplantation in multiple sclerosis patients. J Exp Med 2005; 201:805–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kostense S, Vandenberghe K, Joling J, Van Baarle D, Nanlohy N, Manting E et al Persistent numbers of tetramer+ CD8+ T cells, but loss of interferon‐γ + HIV‐specific T cells during progression to AIDS. Blood 2002; 99:2505–11. [DOI] [PubMed] [Google Scholar]
- 26. Shanker P, Russo M, Harnisch B, Patterson M, Skolnik P, Lieberman J. Impaired function of circulating HIV‐specific CD8+ T cells in chronic human immunodeficiency virus infection. Blood 2000; 96:3094–101. [PubMed] [Google Scholar]
- 27. Gruener NH, Lechner F, Jung MC, Diepolder H, Gerlach T, Lauer G et al Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J Virol 2001; 75:5550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Urbani S, Boni C, Missale G, Elia G, Cavallo C, Massari M et al Virus‐specific CD8+ lymphocytes share the same effector‐memory phenotype but exhibit functional differences in acute hepatitis B and C. J Virol 2002; 76:12423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reignat S, Webster GJM, Brown D, Ogg GS, King A, Seneviratne SL et al Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J Exp Med 2002; 195:1089–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greten TF, Slansky JE, Kubota R, Soldan SS, Jaffee EM, Leist TP et al Direct visualization of antigen‐specific T cells: HTLV‐1 Tax11‐19‐specific CD8+ T cells are activated in peripheral blood and accumulate in cerebrospinal fluid from HAM/TSP patients. Proc Natl Acad Sci USA 1998; 95:7568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol 2007; 19:408–15. [DOI] [PubMed] [Google Scholar]
- 32. Wherry EJ. T cell exhaustion. Nat Immunol 2011; 12:492–9. [DOI] [PubMed] [Google Scholar]
- 33. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H et al Engagement of the PD‐1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000; 192:1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 2007; 8:239–45. [DOI] [PubMed] [Google Scholar]
- 35. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH et al Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006; 439:682–7. [DOI] [PubMed] [Google Scholar]
- 36. Morel E, Bellon T. HLA class I molecules regulate IFN‐γ production induced in NK cells by target cells, viral products, or immature dendritic cells through the inhibitory receptor ILT2/CD85j. J Immunol 2008; 181:2368–81. [DOI] [PubMed] [Google Scholar]
- 37. Chiurchiu V, Cencioni MT, Bisicchia E, De Bardi M, Gasperini C, Borsellino G et al Distinct modulation of human myeloid and plasmacytoid dendritic cells by anandamide in multiple sclerosis. Ann Neurol 2013; 73:626–36. [DOI] [PubMed] [Google Scholar]
- 38. Makedonas G, Hutnick N, Haney D, Amick AC, Gardner J, Cosma G et al Perforin and IL‐2 upregulation define qualitative differences among highly functional virus‐specific human CD8+ T cells. PLoS Pathog 2010; 6(3):e1000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cencioni MT, Chiurchiu V, Catanzaro G, Borsellino G, Bernardi G, Battistini L et al Anandamide suppresses proliferation and cytokine release from primary human T‐lymphocytes mainly via CB2 receptors. PLoS ONE 2010; 5(1):e8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lecoeur H, Fevrier M, Garcia S, Riviere Y, Gougeon ML. A novel flow cytometric assay for quantitation and multiparametric characterization of cell‐mediated cytotoxicity. J Immunol Methods 2001; 253:177–87. [DOI] [PubMed] [Google Scholar]
- 41. Kim GG, Donnenberg VS, Donnenberg AD, Gooding W, Whiteside TL. A novel multiparametric flow cytometry‐based cytotoxicity assay simultaneously immunophenotypes effector cells: Comparisons to a 4 h Cr‐51‐release assay. J Immunol Methods 2007; 325:51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M et al Meningeal B‐cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007; 130:1089–104. [DOI] [PubMed] [Google Scholar]
- 43. Carter LL, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR et al PD‐1: PD‐L inhibitory pathway affects both CD4+and CD8+ T cells and is overcome by IL‐2. Eur J Immunol 2002; 32:634–43. [DOI] [PubMed] [Google Scholar]
- 44. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I et al PD‐L2 is a second ligand for PD‐I and inhibits T cell activation. Nat Immunol 2001; 2:261–8. [DOI] [PubMed] [Google Scholar]
- 45. Ascherio A, Munger KL, Lunemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol 2012; 8:602–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ascherio A, Munger KL. Epstein–Barr virus infection and multiple sclerosis: a review. J Neuroimmune Pharmacol 2010; 5:271–7. [DOI] [PubMed] [Google Scholar]
- 47. Maghzi AH, Marta M, Bosca I, Etemadifar M, Dobson R, Maggiore C, et al Viral pathophysiology of multiple sclerosis: a role for Epstein–Barr virus infection? Pathophysiology 2011; 18:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Torkildsen O, Stansberg C, Angelskar SM, Kooi EJ, Geurts JJG, van der Valk P et al Upregulation of immunoglobulin‐related genes in cortical sections from multiple sclerosis patients. Brain Pathol 2010; 20:720–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Howell OW, Reeves CA, Nicholas R, Carassiti D, Radotra B, Gentleman SM et al Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 2011; 134:2755–71. [DOI] [PubMed] [Google Scholar]
- 50. Abrahamsson SV, Angelini DF, Dubinsky AN, Morel E, Oh U, Jones JL et al Non‐myeloablative autologous haematopoietic stem cell transplantation expands regulatory cells and depletes IL‐17 producing mucosal‐associated invariant T cells in multiple sclerosis. Brain 2013; 136:2888–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Focosi D, Bestagno M, Burrone O, Petrini M. CD57+ T lymphocytes and functional immune. J Leukoc Biol 2010; 87:107–16. [DOI] [PubMed] [Google Scholar]
- 52. Mollet L, Sadat‐Sowti B, Duntze J, Leblond V, Bergeron F, Calvez V et al CD8hi+CD57+ T lymphocytes are enriched in antigen‐specific T cells capable of down‐modulating cytotoxic activity. Int Immunol 1998; 10:311–23. [DOI] [PubMed] [Google Scholar]
- 53. Broux B, Pannemans K, Zhang X, Markovic‐Plese S, Broekmans T, Eijnde BO et al CX(3)CR1 drives cytotoxic CD4+CD28– T cells into the brain of multiple sclerosis patients. J Autoimmun 2012; 38:10–9. [DOI] [PubMed] [Google Scholar]
- 54. Le Priol Y, Puthier D, Lecureuil C, Combadiere C, Debre P, Nguyen C et al High cytotoxic and specific migratory potencies of senescent CD8+ CD57+ cells in HIV‐infected and uninfected individuals. J Immunol 2006; 177:5145–54. [DOI] [PubMed] [Google Scholar]
- 55. Lindsey JW, Hatfield LM, Crawford MP, Patel S. Quantitative PCR for Epstein–Barr virus DNA and RNA in multiple sclerosis. Mult Scler 2009; 15:153–8. [DOI] [PubMed] [Google Scholar]
- 56. Pender MP, Csurhes PA, Lenarczyk A, Pfluger CM, Burrows SR. Decreased T cell reactivity to Epstein–Barr virus infected lymphoblastoid cell lines in multiple sclerosis. J Neurol Neurosurg Psychiatry 2009; 80:498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zivadinov R, Cerza N, Hagemeier J, Carl E, Badgett D, Ramasamy DP et al Humoral response to EBV is associated with cortical atrophy and lesion burden in patients with MS. Neurol Neuroimmunol Neuroinflamm 2016; 3:e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zivadinov R, Chin J, Horakova D, Bergsland N, Weinstock‐Guttman B, Tamano‐Blanco M. et al Humoral responses to herpesviruses are associated with neurodegeneration after a demyelinating event: results from the Multi‐Center SET study. J Neuroimmuno 2014; 273:58–64. [DOI] [PubMed] [Google Scholar]
- 59. Farrell RA, Antony D, Wall GR, Clark DA, Fisniku L, Swanton J et al Humoral immune response to EBV in multiple sclerosis is associated with disease activity on MRI. Neurology 2009; 73:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lunemann JD, Tintore M, Messmer B, Strowig T, Rovira A, Perkal H et al Elevated Epstein–Barr virus‐encoded nuclear antigen‐1 immune responses predict conversion to multiple sclerosis. Ann Neurol 2010; 67:159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang Z, Jin B, Zhang JY, Xu B, Wang H, Shi M et al Dynamic decrease in PD‐1 expression correlates with HBV‐specific memory CD8 T‐cell development in acute self‐limited hepatitis B patients. J Hepatol 2009; 50:1163–73. [DOI] [PubMed] [Google Scholar]
- 62. Peferoen LA, Lamers F, Lodder LN, Gerritsen WH, Huitinga I, Melief J et al Epstein–Barr virus is not a characteristic feature in the central nervous system in established multiple sclerosis. Brain 2010; 133:e137. [DOI] [PubMed] [Google Scholar]
- 63. Lassmann H, Niedobitek G, Aloisi F, Middeldorp JM, NeuroproMiSe EBVWG . Epstein–Barr virus in the multiple sclerosis brain: a controversial issue–report on a focused workshop held in the Centre for Brain Research of the Medical University of Vienna, Austria. Brain 2011; 134:2772–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sauce D, Almeida JR, Larsen M, Haro L, Autran B, Freeman GJ et al PD‐1 expression on human CD8 T cells depends on both state of differentiation and activation status. AIDS 2007; 21:2005–13. [DOI] [PubMed] [Google Scholar]
- 65. Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S et al PD‐1 expression on HIV‐specific T cells is associated with T‐cell exhaustion and disease progression. Nature 2006; 443:350–4. [DOI] [PubMed] [Google Scholar]
- 66. Staron MM, Gray SM, Marshall HD, Parish IA, Chen JH, Perry CJ et al The transcription factor FoxO1 sustains expression of the inhibitory receptor PD‐1 and survival of antiviral CD8+ T cells during chronic infection. Immunity 2014; 41:802–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rinaldo CR Jr, Richter BS, Black PH, Callery R, Chess L, Hirsch MS. Replication of herpes simplex virus and cytomegalovirus in human leukocytes. J Immunol 1978; 120:130–6. [PubMed] [Google Scholar]
- 68. Honess RW, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol 1974; 14:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. (a) Dot plots show the gating strategy used to define immunoglobulin‐like transcript 2 (ILT2) and programmed death‐1 (PD‐1) expression on CD8+ CD57+ T cells. (b) Linear correlation between degranulation of granzyme B (GZMB), perforin (PERF) and interferon‐γ (IFN‐γ) and ILT2 expression on CD8+ CD57+ T cells from 19 healthy donors, Pearson *P < 0·05, **P < 0·01). (c) Dot plot shows the PDL‐1 expression on autologous Epstein–Barr virus (EBV)‐infected lymphoblastoid B cell lines (B‐LCL)
Figure S2. CD8high/low CD57+ are investigated for expression of CD3.
Table S1. Demographic, clinical, autopsy characteristics of the multiple sclerosis cases analysed.


