Summary
Macaca leonina (northern pig‐tailed macaques, NPMs) have variable disease progression during SIVmac239 infection. In the present study, we analysed, for the first time, the correlations between T‐cell phenotypes and disease progression in NPMs during SIVmac239 infection. In comparison to normal progressors (NPs), slow progressors (SPs) had lower chronic T‐cell activation and exhaustion levels. In addition, SPs showed higher peripheral CD4+ T‐cell count and CD4 : CD8 ratio, and lower plasma viral load than NPs. CD4+ T‐cell count and CD4 : CD8 ratio decreased more sharply in NPs than in SPs. Furthermore, T cells in NPs were more highly differentiated, at least in acute infection, than in SPs. These results indicated that T‐cell phenotypes were correlated with disease progression in SIVmac239‐infected NPMs and these correlations may provide valuable guidance for the improvement of therapeutic strategies tested in NPMs.
Keywords: disease progression, Macaca leonina, northern pig‐tailed macaques, SIVmac239, T‐cell phenotype
Abbreviations
- HIV
human immunodeficiency virus
- NPMs
northern pig‐tailed macaques
- NPs
normal progressors
- PD‐1
programmed cell death protein 1
- SHIV
simian‐human immunodeficiency virus
- SIV
simian immunodeficiency virus
- SPs
slow progressors
- Tcm
central memory T
Introduction
Elevated and persistent immune activation is one of the major characteristics induced by human immunodeficiency virus type 1 (HIV‐1) infection.1, 2 Although the cause of immune activation during HIV‐1 infection is not fully understood, persistent immune activation is associated with a poor immunological reconstitution and prognosis.3, 4 It has long been proved that high CD38 or HLA‐DR expression on CD4+ and CD8+ T cells increases morbidity and mortality in HIV disease.5, 6, 7, 8 In addition, we also found a correlation between T‐cell activation and death of simian/human immunodeficiency virus (SHIV) 89.6‐infected Chinese rhesus macaques.9 Programmed cell death protein 1 (PD‐1), a negative regulator of T‐cell activation and biomarker of T‐cell exhaustion is also proved to be associated with disease progression.10 Expression of PD‐1 on CD4+ and CD8+ T cells correlates positively with plasma viral load and negatively with CD4+ T‐cell count.10, 11 In addition, PD‐1 expression on CD4+ and CD8+ T cells is associated with their activation respectively11.
Progressive depletion of CD4+ T cells induced by direct cytotoxicity or other indirect mechanisms is a hallmark of HIV‐1 infection.12 Immune deficiency and the following opportunistic infections are directly associated with low CD4+ T‐cell counts.12 Peripheral CD4+ T‐cell count together with plasma viral load count were used as predictors of AIDS disease progression in earlier years, although they were proved not as predictive as immune activation later.6, 13, 14, 15 Nowadays, peripheral CD4+ T‐cell count and plasma viral load are still selected as significant indicators of clinical disease progression.16, 17 Besides, the CD4 : CD8 ratio is another easily available parameter that is associated with immunological dysfunction and disease progression.18, 19, 20 A persistently low CD4 : CD8 ratio is associated with increased innate and adaptive immune activation and higher risk of morbidity and mortality.20 Except for the parameters mentioned above, pre‐infection level and loss of CD4+ central memory (Tcm) cell may also predict rapid disease progression in Macaca nemestrina.21 Therefore, T‐cell phenotypes and counts may reflect the disease progression to some extent.
Pig‐tailed macaques are valuable HIV/AIDS animal models. Therein, Macaca nemestrina (southern pig‐tailed macaques) are widely used in HIV/AIDS research, while Macaca leonina (northern pig‐tailed macaques, NPMs) are scarcely studied. To establish appropriate HIV/AIDS animal models using NPMs, we infected six NPMs with simian immunodeficiency virus (SIV) mac239, during which NPMs had variable disease progression. In consideration of the evaluation of therapeutic strategies in NPMs, it is significant to study the factors correlated with disease progression. As T‐cell phenotypes were routinely analysed during animal experiments, we decided to study the relationships between disease progression and T‐cell phenotypes, including T‐cell activation and exhaustion, CD4+ T‐cell count, CD4 : CD8 ratio and T‐cell differentiation.
Materials and methods
Animals and sample collection
Six male adult (6–8 years old) NPMs were enrolled in our study. They were healthy and negative for SIV, simian type‐D retrovirus, simian T‐lymphotropic virus and B virus before this study. A tissue culture infectious dose 50% of 3000 SIVmac239 was inoculated into each macaque by intravenous injection. All the macaques were housed in Kunming Primate Research Centre in accordance with the guide of the American Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). All the animal experiment procedures in our study were approved by the Ethics Committee of Kunming Institute of Zoology, Chinese Academy of Sciences (approval number: SYDW‐2015023; approval date: 20 June 2015).
Ketamine hydrochloride was used for the anaesthesia of animals. After complete anaesthesia, peripheral blood was collected through venepuncture and blood was drawn into vacuum tubes containing EDTA. Anaesthetized animals were taken good care of until they awoke.
Plasma viral load
Plasma was isolated by 1000 g centrifugation from peripheral blood and cryopreserved in small aliquots in −80°C. Viral RNA was extracted from plasma using a High Pure Viral RNA Kit (Roche Diagnostics GmbH, Roche Applied Science, Mannheim Germany)22 according to the manufacturer's instructions. Plasma viral loads were determined by real‐time PCR (ABI ViiA™ 7 Real‐Time PCR System; Applied Biosystems, Foster City, CA, USA). Primers, probe and PCR conditions were all the same as previously described.9
Multiparameter flow cytometry
Multiparameter flow cytometry was performed using peripheral blood rather than cryopreserved peripheral blood mononuclear cells. The following anti‐human flow cytometry antibodies cross‐reactive with macaques were used: anti‐CD3‐phycoerythrin (PE) (clone SP34‐2), anti‐CD8‐PE/Cy7 (clone RPA‐T8), anti‐CD3‐allophycocyanin/Cy7 (clone SP34‐2) and anti‐HLA‐DR‐allophycocyanin (clone G46‐6) were purchased from BD Pharmingen, Franklin Lakes, New Jersey, USA; anti‐CD4‐Peridinin chlorophyll protein/Cy5.5 (clone OKT4) was from BioLegend (San Diego, CA, USA); anti‐PD‐1‐PE (clone eBioJ105) was from eBioscience (San Diego, CA, USA). Flow cytometric procedures were described previously.23, 24 Flow cytometric acquisitions were performed on a BD FACSVerse™ (Franklin Lakes, New Jersey, USA) flow cytometer and all flow cytometric data were analysed on flowjo software (vX.0.7; TreeStar, Ashland, OR, USA).
Statistical analysis
Two‐way analysis of variance compared the difference of T‐cell activation/exhaustion, peripheral CD4+ T‐cell count, CD4 : CD8 ratio and plasma viral load between normal and slow progressor NPMs. P < 0·05 was considered statistically significant. All data analyses were performed using the graphpad prism software (version 6.01; GraphPad Software, La Jolla, CA, USA).
Results
The disease progression varies between SIVmac239‐infected NPMs
Two individuals (08247 and 09211) showed a wasting syndrome (weight loss > 10% versus day 0 for at least 1 month) among six SIVmac239‐infected NPMs (Table 1).25 They died on day 491 and day 476 post infection, respectively, and showed obvious pathological changes in tissues. Two NPMs (08247 and 08287) showed thrombocytopenia during the infection. The other three NPMs (09203, 10205 and 10225) did not show wasting syndrome or thrombocytopenia. Based on the AIDS symptoms, we divided the six NPMs into two groups: normal progressors (NPs) and slow progressors (SPs).
Table 1.
Disease progression status of simian immunodeficiency virus mac239‐infected northern pig‐tailed macaques
| Group | Macaque | Death time (days post infection) | AIDS symptoms |
|---|---|---|---|
| Normal progressors | 08247 | 491 | Wasting syndromea, thrombocytopeniab, lymphadenopathy, lung lesions, liver lesions |
| 08287 | NDc | Thrombocytopenia | |
| 09211 | 476 | Wasting syndrome, lung lesions | |
| Slow progressors | 09203 | ND | No wasting syndrome, no thrombocytopenia, alive |
| 10205 | ND | ||
| 10225 | ND |
Wasting syndrome is defined as weight loss > 10% for at least 1 month.
Thrombocytopenia is defined as < 100 × 109 platelets/l blood.
ND, not dead.
Chronic T‐cell activation and exhaustion are associated with disease progression
We analysed T‐cell activation (defined by HLA‐DR expression) and exhaustion (defined by PD‐1 expression) in a long‐term infection. The infection was divided into acute phase and chronic phase on the 12th week post infection. Expressions/co‐expressions of these markers showed similar levels in NPs and SPs in acute phase (Fig. 1). These results implied that acute T‐cell activation and exhaustion might not affect disease progression. Conversely, chronic T‐cell activation and exhaustion levels in NPs were higher than in SPs, with statistical significance, except CD8+ T‐cell exhaustion (P = 0·6158) (Fig. 1b). Overall, there were strong relationships between chronic T‐cell activation/exhaustion and disease progression.
Figure 1.
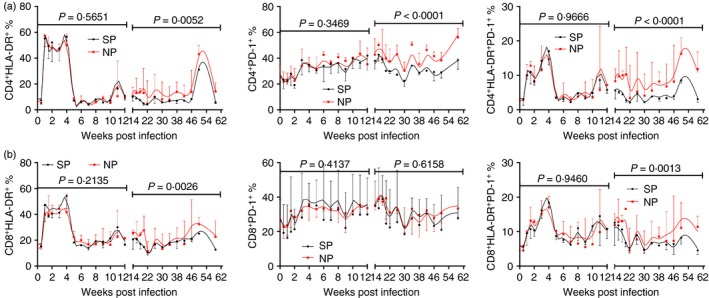
Comparisons of T‐cell activation and exhaustion levels between normal progressor (NP) and slow progressor (SP) northern pig‐tailed macaques (NPMs). Longitudinal analysis of CD38, HLA‐DR and PD‐1 expression/co‐expression on (a) CD4+ T cells and (b) CD8+ T cells. The infection was divided into acute phase and chronic phase at 12 weeks post infection. Data are shown as median and error. Two‐way analysis of variance compared the difference of activation and exhaustion marker expressions between normal and slow progressor NPMs. P < 0·05 was considered as statistically significant.[Colour figure can be viewed at wileyonlinelibrary.com]
Disease progression was correlated with peripheral CD4+ T‐cell count, CD4 : CD8 ratio and plasma viral load
Peripheral CD4+ T‐cell count and plasma viral load are clinically conventional surrogate markers of HIV‐1 disease progression.26 In addition, it has been reported that low CD4 : CD8 ratio predicts disease progression.19, 20 Therefore, we analysed the relationships between disease progression and peripheral CD4+ T cell, CD4 : CD8 ratio and plasma viral load in our study. Peripheral CD4+ T‐cell count and CD4 : CD8 ratio were higher in SPs than in NPs throughout the infection whereas plasma viral load was lower in SPs than in NPs during chronic infection (Fig. 2). These results indicated that peripheral CD4+ T‐cell count, CD4 : CD8 ratio and plasma viral load might be appropriate surrogate markers of disease progression.
Figure 2.
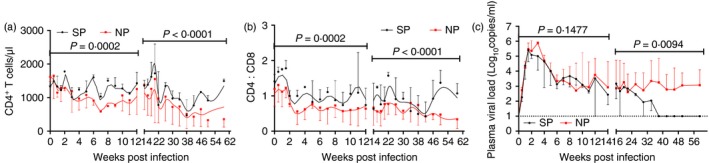
Comparisons of peripheral CD4+ T‐cell count, CD4 : CD8 ratio and plasma viral load between normal progressor (NP) and slow progressor (SP) northern pig‐tailed macaques (NPMs). Longitudinal analysis of (a) peripheral CD4+ T‐cell count, (b) CD4 : CD8 ratio and (c) plasma viral load. Data are shown as median and error. The infection was divided into acute phase and chronic phase at 12 weeks post infection. Two‐way analysis of variance compared the difference of peripheral CD4+ T‐cell count, CD4 : CD8 ratio and plasma viral load between normal and slow progressor NPMs. P < 0·05 was considered as statistically significant. [Colour figure can be viewed at wileyonlinelibrary.com]
Disease progression was correlated with fold changes of peripheral CD4+ T‐cell count and CD4 : CD8 ratio
Given the individual differences of pre‐infection peripheral CD4+ T‐cell count and CD4 : CD8 ratio, we further compared the fold changes of these parameters in different groups. Peripheral CD4+ T‐cell count decreased more sharply in NPs than in SPs in both acute and chronic phases (Fig. 3a). In addition, peripheral CD4+ T‐cell count in SPs recovered to pre‐infection levels, but not in NPs. The CD4 : CD8 ratio also decreased more severely in NPs than in SPs throughout the infection (Fig. 3b). These results further implied that peripheral CD4+ T‐cell count and CD4 : CD8 ratio were potential surrogate markers of disease progression.
Figure 3.
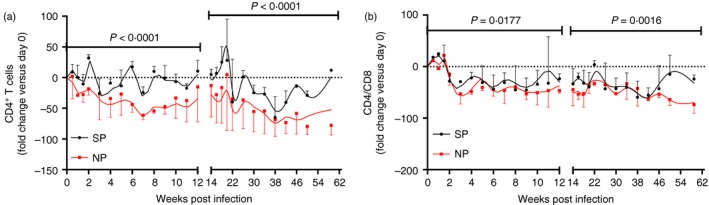
Fold changes of peripheral CD4+ T‐cell count and CD4 : CD8 ratio between normal progressor (NP) and slow progressor (SP) northern pig‐tailed macaques (NPMs). Longitudinal analysis of fold changes of (a) peripheral CD4+ T‐cell count and (b) CD4 : CD8 ratio. Fold change of day . Data are shown as median and error. The infection was divided into acute phase and chronic phase at 12 weeks post infection. Two‐way analysis of variance compared the fold changes of peripheral CD4+ T‐cell count and CD4 : CD8 ratio between normal and slow progressor NPMs. P < 0·05 was considered as statistically significant. [Colour figure can be viewed at wileyonlinelibrary.com]
High differentiation of T cells may predict rapid disease progression
It has been proved that loss of CD4+ Tcm cells may predict rapid disease progression in Macaca nemestrina.21 Whether it is the same in Macaca leonina is still unclear. Therefore, we compared the frequencies of T‐cell subsets in different groups. There was no obvious difference in frequencies of CD4+ naive T cells (Fig. 4a). But, the frequency of CD4+ Tcm cells in SPs was higher than in NPs during acute infection, whereas the frequency of CD4+ T effector memory cells was just the opposite. The differences no longer lasted during chronic infection. Although there was no significant difference in the frequencies of CD4+ Tcm cells, we could still infer the more severe loss of CD4+ Tcm cells in NPs from the CD4+ T‐cell count and fold change. The frequency of CD8+ naive T cells in SPs was higher than in NPs throughout the infection, whereas the frequency of CD8+ T effector memory cells was just the opposite (Fig. 4b). The frequency of CD8+ Tcm cells in SPs was higher than in NPs during acute infection, but not in chronic infection. These results indicated that high differentiation of T cells, at least in acute infection, might predict rapid disease progression.
Figure 4.
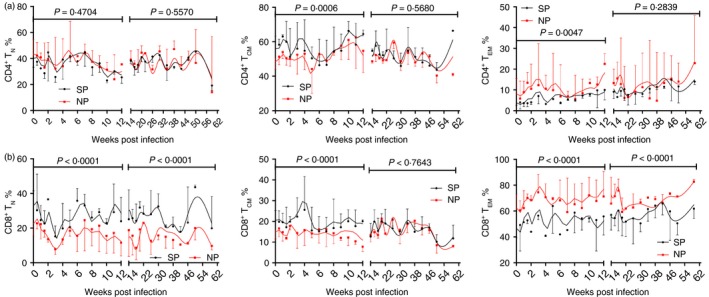
Frequencies of peripheral CD4+ T‐cell subsets. CD4+ T cells were divided into naive T cells (CD28+ CD95−), central memory T cells (CD28+ CD95+) and effector memory T cells (CD28− CD95+). Longitudinal analysis of frequencies of (a) CD4+ T‐cell subsets and (b) CD8+ T‐cell subsets. Data are shown as median and error. The infection was divided into acute phase and chronic phase at 12 weeks post infection. Two‐way analysis of variance compared the frequencies of CD4+ T‐cell subsets and CD8+ T‐cell subsets between normal progressor (NP) and slow progressor (SP) northern pig‐tailed macaques. P < 0·05 was considered as statistically significant. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
Predictors of disease progression may provide valuable guidance for the improvement of therapeutic strategies tested in HIV/AIDS animal models. Here we studied the relationships between disease progression and various T‐cell phenotypes in NPMs during SIVmac239 infection. Overall, NPs showed higher chronic T‐cell activation and exhaustion levels than SPs. Meanwhile, SPs showed higher peripheral CD4+ T‐cell count and CD4 : CD8 ratio, and lower plasma viral load. In addition, T cells were more highly differentiated in NPs than in SPs.
Although CD4+ T‐cell counts in SPs were higher and viral loads in SPs were lower than that of NPs, we still cannot eliminate the irregularity of the results. Hence, we further analysed the correlations between T‐cell activation/exhaustion and CD4+ T‐cell count, CD4 : CD8 ratio and plasma viral load (see Supplementary material, Fig. [Link], [Link], [Link]). Overall, T‐cell activation/exhaustion is negatively correlated with CD4+ T‐cell count and CD4 : CD8 ratio and positively correlated with plasma viral load. But, the correlations are not consistent in different T‐cell subsets or different infection phases. As T‐cell activation has been proved to be more predictive,6, 15 CD4+ T‐cell count and viral load may not be as prognostic as the results show. In addition, the correlations between CD4 : CD8 ratio and T‐cell exhaustion are more consistent than that of CD4+ T‐cell count and viral load (see Supplementary material, Fig. [Link], [Link], [Link]), which indicates that CD4 : CD8 is more prognostic, as previously reported.20
Although chronic T‐cell activation/exhaustion were proved to be correlated with rapid disease progression in SIVmac239‐infected NPMs, T‐cell activation and exhaustion are complicated states during which a multitude of markers are increasingly expressed.27, 28, 29 More markers may be needed to show the accurate correlations between T‐cell activation/exhaustion and disease progression.
As disease progression is controlled by many factors, more relative immunological parameters may be better for the estimation of disease progression. Thrombocytopenia is used as a factor for the classification of different progression groups, but we also compared the fold change of platelets during SIV infection. The results indicated that a sharp decrease of platelets in chronic infection might predict rapid disease progression in SIVmac239‐infected NPMs (see Supplementary material, Fig. S2). In addition, to precisely prognosticate the disease progression, more predictors such as coagulation biomarkers, microbial translocation‐related factors, thrombocytopenia and plasma cytokine levels may provide complements to future studies.30, 31, 32, 33
Unfortunately, there are also some limitations in our study. First, the small number of NPMs enrolled in our study may not fully reflect the relationships between T‐cell phenotypes and disease progression. Second, to thoroughly analyse the correlations between disease progression and T‐cell phenotypes, more phenotypes other than activation/exhaustion and differentiation should be involved and a comprehensive analysis may provide a profound insight into the correlations. Finally, as large numbers of HIV‐infected patients are receiving treatment, the correlations between T‐cell phenotypes and disease progression in treated NPMs may provide more valuable suggestions for the improvement of therapeutic strategyies.
In summary, we investigated the correlations between disease progression and T‐cell phenotypes in SIVmac239‐infected NPMs. The results indicated that T‐cell phenotypes might predict disease progression to some extent. When therapeutic strategies were tested in NPMs, T‐cell phenotypes might be potentially appropriate indicators of effectiveness.
Disclosures
The authors participating in this study declare that there are no conflicts of interest regarding the publication of this article.
Supporting information
Figure S1. Correlations between T‐cell activation/exhaustion and (a) peripheral CD4+ T‐cell count, (b) plasma viral load and (c) CD4 : CD8 ratio.
Figure S2. Fold change of platelets.
Acknowledgements
This work was partly supported by grants from the National Natural Science Foundation of China (81471620; 81671627; 81571606; U1604287; 81601808; 81172876; U0832601), the National Basic Research Programme of China (2012CBA01305), and the Knowledge Innovation Programme of the Chinese Academy of Sciences (CASIMM0320163020; KSCX2‐EW‐R‐13), and Yunnan Applicative and Basic Research Programme (2014FB181; 201501PH00015; C0120150617). The work has not been presented at any meetings.
Reference
- 1. Klatt NR, Chomont N, Douek DC, Deeks SG. Immune activation and HIV persistence: implications for curative approaches to HIV infection. Immunol Rev 2013; 254:326–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hatano H. Immune activation and HIV persistence: considerations for novel therapeutic interventions. Curr Opin HIV AIDS 2013; 8:211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lederman MM, Calabrese L, Funderburg NT, Clagett B, Medvik K, Bonilla H et al Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis 2011; 204:1217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodger AJ, Fox Z, Lundgren JD, Kuller LH, Boesecke C, Gey D et al Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. J Infect Dis 2009; 200:973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu Z, Cumberland WG, Hultin LE, Kaplan AH, Detels R, Giorgi JV. CD8+ T‐lymphocyte activation in HIV‐1 disease reflects an aspect of pathogenesis distinct from viral burden and immunodeficiency. J Acquir Immune Defic Syndr Hum Retrovirol 1998; 18:332–40. [DOI] [PubMed] [Google Scholar]
- 6. Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP et al Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis 1999; 179:859–70. [DOI] [PubMed] [Google Scholar]
- 7. Hazenberg MD, Otto SA, van Benthem BHB, Roos MTL, Coutinho RA, Lange JMA et al Persistent immune activation in HIV‐1 infection is associated with progression to AIDS. AIDS 2003; 17:1881–8. [DOI] [PubMed] [Google Scholar]
- 8. Hatano H, Jain V, Hunt PW, Lee TH, Sinclair E, Do TD et al Cell‐based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD‐1)‐expressing CD4+ T cells. J Infect Dis 2013; 208:50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tian RR, Zhang MX, Zhang LT, Zhang XL, Zheng HY, Zhu L et al High immune activation and abnormal expression of cytokines contribute to death of SHIV89.6‐infected Chinese rhesus macaques. Arch Virol 2015; 160:1953–66. [DOI] [PubMed] [Google Scholar]
- 10. Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S et al PD‐1 expression on HIV‐specific T cells is associated with T‐cell exhaustion and disease progression. Nature 2006; 443:350–4. [DOI] [PubMed] [Google Scholar]
- 11. Cockerham LR, Jain V, Sinclair E, Glidden DV, Hartogenesis W, Hatano H et al Programmed death‐1 expression on CD4+ and CD8+ T cells in treated and untreated HIV disease. AIDS 2014; 28:1749–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Okoye AA, Picker LJ. CD4+ T‐cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev 2013; 254:54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. deWolf F, Spijkerman I, Schellekens PT, Langendam M, Kuiken C, Bakker M et al AIDS prognosis based on HIV‐1 RNA, CD4+ T‐cell count and function: markers with reciprocal predictive value over time after seroconversion. AIDS 1997; 11:1799–806. [DOI] [PubMed] [Google Scholar]
- 14. Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P et al Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV‐1 infection. Ann Intern Med 1997; 126:946–54. [DOI] [PubMed] [Google Scholar]
- 15. Liu ZY, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the multicenter AIDS cohort study than CD4+ cell count, soluble immune activation markers, or combinations of HLA‐DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol 1997; 16:83–92. [DOI] [PubMed] [Google Scholar]
- 16. Kelley CF, Kitchen CMR, Hunt PW, Rodriguez B, Hecht FM, Kitahata M et al Incomplete peripheral CD4+ cell count restoration in HIV‐infected patients receiving long‐term antiretroviral treatment. Clin Infect Dis 2009; 48:787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Robbins GK, Spritzler JG, Chan ES, Asmuth DM, Gandhi RT, Rodriguez BA et al Incomplete reconstitution of T cell subsets on combination antiretroviral therapy in the AIDS clinical trials group protocol 384. Clin Infect Dis 2009; 48:350–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hema MN, Ferry T, Dupon M, Cuzin L, Verdon R, Thiebaut R et al Low CD4/CD8 ratio is associated with non aids‐defining cancers in patients on antiretroviral therapy: ANRS CO8 (Aproco/Copilote) prospective cohort study. PLoS ONE 2016; 11:e0161594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mussini C, Lorenzini P, Cozzi‐Lepri A, Lapadula G, Marchetti G, Nicastri E et al CD4/CD8 ratio normalisation and non‐AIDS‐related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV 2015; 2:e98–106. [DOI] [PubMed] [Google Scholar]
- 20. Serrano‐Villar S, Sainz T, Lee SA, Hunt PW, Sinclair E, Shacklett BL et al HIV‐infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non‐AIDS morbidity and mortality. PLoS Pathog 2014; 10:e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mason RD, De Rose R, Seddiki N, Kelleher AD, Kent SJ. Low pre‐infection levels and loss of central memory CD4+ T cells may predict rapid progression in SIV‐infected pigtail macaques. Virology 2008; 381:11–5. [DOI] [PubMed] [Google Scholar]
- 22. Badley AD, Dockrell DH, Algeciras A, Ziesmer S, Landay A, Lederman MM et al In vivo analysis of Fas/FasL interactions in HIV‐infected patients. J Clin Invest 1998; 102:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xia HJ, Zhang GH, Wang RR, Zheng YT. The influence of age and sex on the cell counts of peripheral blood leukocyte subpopulations in Chinese Rhesus Macaques. Cell Mol Immunol 2009; 6:433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zheng HY, Zhang MX, Pang W, Zheng YT. Aged Chinese rhesus macaques suffer severe phenotypic T‐ and B‐cell aging accompanied with sex differences. Exp Gerontol 2014; 55:113–9. [DOI] [PubMed] [Google Scholar]
- 25. Wanke CA, Silva M, Knox TA, Forrester J, Speigelman D, Gorbach SL. Weight loss and wasting remain common complications in individuals infected with human immunodeficiency virus in the era of highly active antiretroviral therapy. Clin Infect Dis 2000; 31:803–5. [DOI] [PubMed] [Google Scholar]
- 26. Hoffmann M, Pantazis N, Martin GE, Hickling S, Hurst J, Meyerowitz J et al Exhaustion of activated CD8 T cells predicts disease progression in primary HIV‐1 infection. PLoS Pathog 2016; 12:e1005661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lichtfuss GF, Hoy J, Rajasuriar R, Kramski M, Crowe SM, Lewin SR. Biomarkers of immune dysfunction following combination antiretroviral therapy for HIV infection. Biomark Med 2011; 5:171–86. [DOI] [PubMed] [Google Scholar]
- 28. Nixon DE, Landay AL. Biomarkers of immune dysfunction in HIV. Curr Opin HIV AIDS 2010; 5:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wherry EJ. T cell exhaustion. Nat Immunol 2011; 12:492–9. [DOI] [PubMed] [Google Scholar]
- 30. Pandrea I, Cornell E, Wilson C, Ribeiro RM, Ma D, Kristoff J et al Coagulation biomarkers predict disease progression in SIV‐infected nonhuman primates. Blood 2012; 120:1357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE et al Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alcantara S, Reece J, Amarasena T, Rose RD, Manitta J, Amin J et al Thrombocytopenia is strongly associated with simian AIDS in pigtail macaques. J Acquir Immune Defic Syndr 2009; 51:374–9. [DOI] [PubMed] [Google Scholar]
- 33. Roberts L, Passmore JAS, Williamson C, Little F, Bebell LM, Mlisana K et al Plasma cytokine levels during acute HIV‐1 infection predict HIV disease progression. AIDS 2010; 24:819–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Correlations between T‐cell activation/exhaustion and (a) peripheral CD4+ T‐cell count, (b) plasma viral load and (c) CD4 : CD8 ratio.
Figure S2. Fold change of platelets.


