Figure 5.
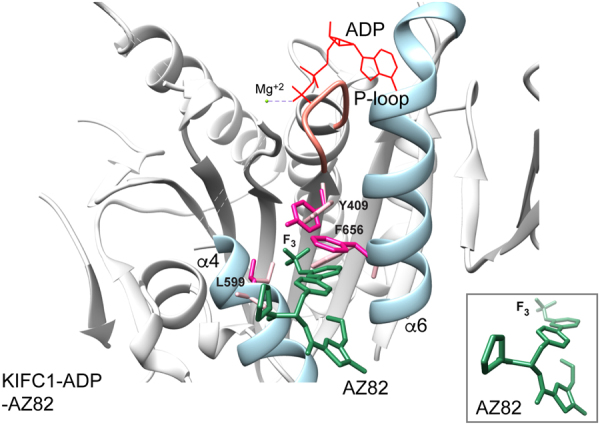
AZ82 docked into the KIFC1 (PDB 5WDH) α4/α6 cleft. AZ82 binding to the KIFC1 α4/α6 cleft gave a predicted free energy change of ΔG = −8.1 kcal/mol. The predicted free energy changes for AZ82 binding to KIFC3 (ΔG = −5.4 kcal/mol) and Ncd (H1, ΔG = −7.9 kcal/mol; H2, ΔG = −6.5 kcal/mol) indicated less favorable binding. The lower predicted free energy change for AZ82 binding to Ncd head H2 than H1 indicates that the drug is unlikely to specifically inhibit Ncd motor-MT activity, as observed for KIFC1. In addition, structural features of AZ82 binding to KIFC3 and Ncd (see Supplementary Fig. S5 for Ncd H1), indicate that the drug may not be stably bound. KIFC1 residues Y409, L599 and F656 are shown as stick models before (pale pink) and after (dark pink) docking. AZ82, dark green; KIFC1 helices α4 and α6, pale blue; P-loop, coral; ADP, red; Mg+2, yellow green. Inset, AZ82 conformation after docking.
