Fig. 1.
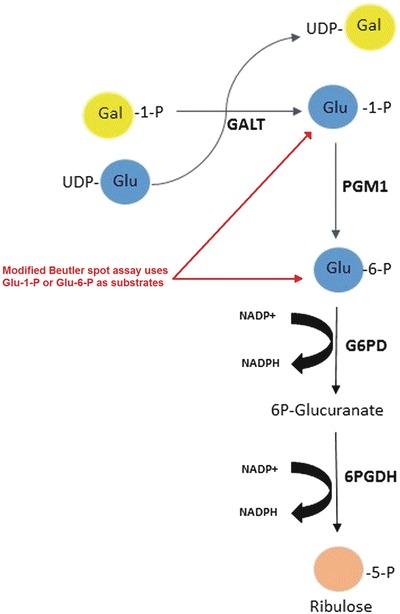
In the Beutler spot assay, the substrates Gal-1-P and uridine diphosphate glucose (UDP-glucose) are part of the test reagent and breakdown into glucose-1-phosphate in the presence of GALT. Glucose-1-phosphate is then further metabolized, stepwise, to ribulose-5-phosphate by PGM1, G6PD, and 6PGD. The last two reactions are in conjunction with the reduction of NADP+ to NADPH. The fluorescence of NADPH is measured to determine GALT deficiency. The Beutler spot assay can be modified to measure G6PD enzyme activity instead of GALT. This was achieved in our laboratory by adding glucose-6-phosphate as the main substrate. Glucose-6-phosphate dehydrogenase (G6PDH) catalyzes the conversion of glucose-6-phosphate to 6-phosphogluconate. In presence of NADP, glucose-6-phosphate is oxidized by G6PD to generate 6-phosphogluconate. This reaction also generates NADPH by the reduction of NADP. The formation of NADPH produces fluorescence, which magnitude is proportional to the G6PD enzyme activity. By using glucose-6-phosphate, the preceding GALT and PGM1 enzymes present/involved in the pathway are avoided and G6PD enzyme is directly measured. This technique has been described previously (Beutler 1994)
