Abstract
Objective:
Probiotics such as lactobacilli prevent the development of a wide range of human and animal's pathogens. The aim of this study was evaluation of antagonistic effect of isolated lactobacilli from local dairy products against three standard strains of Staphylococcus aureus, Bacillus subtilis, and Pseudomonas aeruginosa.
Materials and Methods:
Twenty samples of local dairy products including cow milk, buffalo milk, cheese, and yogurt were collected from different areas of Ahwaz city. Antimicrobial disc diffusion method was applied on S. aureus (ATCC-6538), B. subtilis (ATCC-12711), and P. aeruginosa (ATCC-27853). Antimicrobial effects of isolates were evaluated by disc diffusion test on Mueller-Hinton agar medium plated with three pathogens.
Results:
Obtained results showed that only three strains of isolated lactobacilli of local dairy samples had inhibitory effects on understudy pathogens including Lactobacillus alimentarius, Lactobacillus sake, and Lactobacillus collinoides. All three isolates showed moderate activity (inhibition zone <15 mm) except of L. collinoides and L. alimentarius that had relatively strong activity (inhibition zone ≥15 mm) against P. aeruginosa and B. subtilis, respectively.
Conclusion:
These bacteria can be raised for the production of various kinds of food, pharmaceutical products, and functional foods.
Keywords: Bacteria, Iran, Lactobacillus, probiotics
INTRODUCTION
Probiotics are living organisms that are used as food additives with beneficial effects on the healthy body by setting microbial balance in gastrointestinal tract.[1,2] Lactic acid bacteria (LAB) as protective cultures are common probiotic organisms that are considered safe due to having specific characteristics. Main genera of LAB are Leuconostoc, Enterococcus, Lactobacillus, Lactococcus, Bifidobacterium, Pediococcus, and Streptococcus.[3] These bacteria cause reduction of gastrointestinal diseases by increasing benefit microorganisms' growth and reducing pathogens' population mechanisms.[4] LAB are widely distributed in the environment that can prevent the growth of pathogenic microorganisms by producing particular substances.[4] According to scientific reports, antiallergic and anticancer effects, increasing fat loss and immune response of the host, improvement symptoms of irritable bowel syndrome, intestinal inflammation, and antibiotic-induced diarrhea are other useful effects of probiotics.[5,6]
Nowadays, probiotics are used not only as a driver of growth but also as a stimulator of the immune system and prevention of many diseases.[7] Food probiotic products because of their nutritional value and health sector over the past parallel to the therapeutic effects are taken into consideration.[8,9] Lactobacilli are Gram-positive and catalase-negative bacteria that are known as most important probiotics and desirable gut microflora.[6] They are normal flora of mouth, intestine, and female genital tract with important role in the control of undesirable microorganisms that can be considered as natural biopreservatives.[4]
Lactobacilli have an important role in controlling undesirable microflora in the gut and are able to prevent the rise of pathogenic bacteria by producing antimicrobial metabolites. They can be used as biological preservatives and are raised naturally in foods.[10]
Antimicrobial activity, bile salts, and acid tolerance are three important features for screening probiotic potential of bacteria that can be used as medical.[2,11] LAB produce organic acids, hydrogen peroxide, diacetyl, bacteriocins, and antifungal compounds such as fatty acids during lactic fermentation. Bacteriocins are protein compounds with growth inhibition ability of sensitive pathogenic bacteria and different degradation system in digestive system compared with antibiotics.[4,11] LAB are resistant to lysozyme, gastric acid, gastrointestinal juice, and bile salts. Antimicrobial compounds are also prepared from them to compete and inhibit pathogenic microorganisms.[4] Such compounds may affect metabolism or toxins of pathogenic bacteria.[12,13]
LAB have protective effects in dairy products against harmful bacteria. Several studies have been done on antibacterial effects of probiotics against Gram-negative and positive bacteria such as Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus.[14,15] The aim of this study was evaluating antagonistic effect of isolated lactobacilli from local dairy products against three standard strains of S. aureus, Bacillus subtilis, and P. aeruginosa.
MATERIALS AND METHODS
Twenty samples of local dairy products including cow milk, buffalo milk, cheese, and yogurt were collected from different areas of Ahwaz city, center of Khuzestan province of Iran. Eight strains of Lactobacillus cultures were isolated and identified by biochemical tests.
Biochemical tests such as Gram stain, catalase, oxidase, indole production and motion study, growth at different temperatures (15–37–45), and various fermentation of sugars (sucrose, lactose, maltose, trehalose, galactose, arabinose, mannitol, fructose, and salicin) were studied.[16]
To isolate and proliferate Lactobacillus cultures, MRS Broth (Merck, Germany), MRS Agar (Merck, Germany), and Mueller-Hinton agar (MHA) media (Merck, Germany) were used.
Isolation and Identification of Lactobacillus (lactic acid bacteria)
Two grams of each sample was transferred in a flask containing MRS Broth as enrichment media and added distilled water to 100 ml and incubated in 37°C. After 24 h, 100 μl of enriched samples was spread on MRS agar and incubated at 37°C and anaerobic condition for 48 h. Bacterial colonies were purified by subsequent subcultures.
Final identification was done using classic microbiology tests including Gram-staining for detecting morphology, catalase and oxidase tests, motility, indole producing, growth at 15°C, and carbohydrates fermentation (arabinose, fructose, galactose, lactose, mannitol, salicin, sucrose, and trehalose) test.[17]
All Gram-positive and catalase-negative bacilli were selected for the assessment of antimicrobial ability. Antimicrobial effect of isolates was evaluated by disc diffusion test on MHA medium plated with three pathogens. For this purpose, fresh culture of isolates was centrifuged (8000 rpm, 15 min) and supernatants were removed. Blank discs were inoculated with 40 μl supernatant of each isolate and were placed on separate MHA medium inoculated with S. aureus (ATCC-6538), B. subtilis (ATCC-12711), and P. aeruginosa (ATCC-27853) strains. Growth inhibition zones of pathogens and isolated lactobacilli inhibitory ability were assessed after incubation of all agar media at 37°C for 24 h.[18]
RESULTS
Obtained results showed that only three strains of isolated lactobacilli of local dairy samples had inhibitory effects on understudy pathogens including L. alimentarius, L. sake, and L. collinoides. Biochemical characteristics of these strains are shown in Table 1.
Table 1.
Biochemical characteristics of three isolated Lactobacillus strains

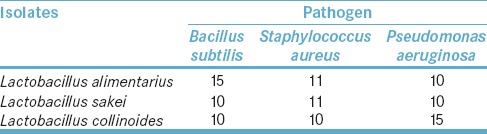
Seventy percent of isolated lactobacilli showed antimicrobial effects on selected pathogens, but inhibitory effects of three strains were more considerable than others [Figure 1]. Growth inhibition zone of three Lactobacillus strains including L. alimentarius, L. sake, and L. collinoides is shown in Table 2.
Figure 1.
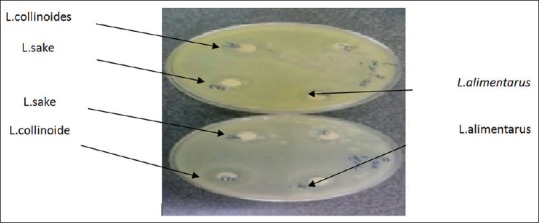
Inhibition zone of three Lactobacillus strains on the Pseudomonas aeruginosa and Bacillus subtilis
Table 2.
Growth inhibition zone diameter (mm) of pathogens with three Lactobacillus strains
DISCUSSION
It is a long time that scientists are trying to substitute synthetic drugs with natural products.[19,20,21,22] Nowadays, various natural materials and methods are used to prevent or treat diseases.[22,23,24,25,26,27,28] The use of probiotics is one of these methods. Lactobacilli and bifidobacteria are normal intestinal flora which by preventing intestinal infection, lowering cholesterol, stimulating the immune system, and reducing the risk of colon cancer play an important role in human health. Probiotic bacteria produce lactic acid and organic acids, reduce the pH environment, and try to prevent the growth of many bacteria. These bacteria produce antimicrobial compounds such as bacteriocin which can be used as natural preservatives.[29]
In this study, it was found that the metabolites produced by these bacteria, which were isolated by centrifugation, were able to prevent the growth of pathogenic bacteria.
The study of Boris et al. showed that lactobacilli strains isolated from dairy products were able to inhibit the growth of P. aeruginosa, E. coli, Salmonella typhimurium, and S. aureus, the latter was in the highest inhibitory effect.[30]
In the study of Ogunbanwo et al., the microbial activity and bacteriocin production of probiotic strains of Lactococcus plantarum and Lactobacillus brevis were searched on multiple pathogens in which the highest inhibitory effect was observed on Bacillus cereus.[31] Coconnier et al. reported that consumption of supernatant (culture supernatant) bacteria Lactobacillus fermentum, Lactobacillus casei, Lactobacillus acidophilus, and Lactococcus lactis had inhibitory effects on a wide range of disease-causing bacteria.[32] Based on the results of the present study, L. alimentarius had the most effect on L. collinoides the highest inhibitory effect on P. aeruginosa and S. aureus showed the greatest effect on L. sake.
Antagonistic activity of three selected isolated lactobacilli of some dairy products was evaluated in this study. The obtained results [Table 2] showed that all three isolates had moderate activity (inhibition zone <15 mm) except L. collinoides and L. alimentarius that had relatively strong activity (inhibition zone ≥15 mm) against P. aeruginosa and B. subtilis, respectively. Antimicrobial effect of lactobacilli against pathogens is mainly due to the production of organic acids and pH reduction in coculture with pathogenic bacteria although they can produce some other substances.[33]
For many years, dairy products have been recognized as valuable products to human health.[34] In recent years, many scientists have isolated and identified LAB and lactobacilli from traditional products worldwide and have evaluated their antagonistic effects against various pathogens.[35] Microorganisms such as lactobacilli and many other bacteria can eliminate pathogens through multiple mechanisms including competitive elimination that results in food safety.[36]
CONCLUSION
Given the results of this study, antagonistic effects of produced substances by the bacteria on a wide range of microorganisms have an important role in food preservation and human health. These bacteria can be raised for the production of various kinds of food and pharmaceutical products. They can also be used for the production of new functional foods. Therefore, increasing use of dairy products containing probiotics, identification and production of foods containing highest and most effective lactobacilli are recommended in daily diet.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflflicts of interest.
REFERENCES
- 1.FAO/WHO. Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria: Report of a Joint FAO/WHO Expert Consultation 2006. 2008 Nov 25; [Google Scholar]
- 2.Hassanzadazar H, Ehsani A, Mardani K, Hesari J. Investigation of antibacterial, acid and bile tolerance properties of lactobacilli isolated from Koozeh cheese. Vet Res Forum. 2012;3:181–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Tafvizi F, Tajabadi Ebrahimi M, Khajareh L. Study genotypic and phylogenetic bacteriocin-producing lactobacilli isolated from dairy product to local and traditional food. J Fasa Univ Med Sci. 2012;2:84. [Google Scholar]
- 4.Hawaz E. Isolation and identification of probiotic lactic acid bacteria from curd and in vitro evaluation of its growth inhibition activities against pathogenic bacteria. Afr J Microbiol Res. 2014;8:1919–425. [Google Scholar]
- 5.Shokryazdan P, Sieo CC, Kalavathy R, Liang JB, Alitheen NB, Faseleh Jahromi M, et al. Probiotic potential of Lactobacillus strains with antimicrobial activity against some human pathogenic strains. Biomed Res Int. 2014;2014:927268. doi: 10.1155/2014/927268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nsofor CA, Sarah U, Chinyere U. Isolation and characterization of lactic acid bacteria from ogi sold in Elele Nigeria. J Biol Food Sci Res. 2014;3:19–22. [Google Scholar]
- 7.Del Piano, Ballare M, Montino M, Orsello F, Garello M, Sforza E. F. Clinical experience with probiotics in the elderly on total enteral nutrition. J Clin Gastroenterol. 2004;38:S111–S4. doi: 10.1097/01.mcg.0000128937.32835.7c. [DOI] [PubMed] [Google Scholar]
- 8.Smid EJ, van Enckevort FJ, Wegkamp A, Boekhorst J, Molenaar D, Hugenholtz J, et al. Metabolic models for rational improvement of lactic acid bacteria as cell factories. J Appl Microbiol. 2005;98:1326–31. doi: 10.1111/j.1365-2672.2005.02652.x. [DOI] [PubMed] [Google Scholar]
- 9.Islam T, Sabrin F, Islam E, Billah M, Islam Didarul KM. Analysis of antimicrobial activity of Lactobacillus paracasei ssp paracasei-1 isolated from regional yogurt. J Microbiol Biotechnol Food Sci. 2012;7:80–9. [Google Scholar]
- 10.Oyetyo VO. Phenotypic characterization and assessment of the inhibitory potential of lactobacillus isolates from different sources. Afr J Biotechnol. 2004;3:355–7. [Google Scholar]
- 11.Salehi M. Antagonistic effect of lactobacilli isolated from native food. J Food Sci Technol Innov. 2012;5:1. [Google Scholar]
- 12.Rushdy Abeer A, Gomaa Zakaria E. antimicrobial compounds produced by probiotic Lactobacillus brevis isolated dairy products. J Am Microbiol. 2013;63:81–90. [Google Scholar]
- 13.Osuntoki A, korie I. Antiooxidant activity of whey from milk fermented with Lactobacillus species isolated from Nigerion fermented foods. J Food Technol Biotechnol. 2010;48:505–11. [Google Scholar]
- 14.Sharafi H, Maleki H, Ahmadian G, Shahbani Zahiri H, Sajedinejad N, Houshmand B, et al. Antibacterial activity and probiotic potential of Lactobacillus plantarum HKN01: A new insight into the morphological changes of antibacterial compound-treated Escherichia coli by electron microscopy. J Microbiol Biotechnol. 2013;23:225–36. doi: 10.4014/jmb.1208.08005. [DOI] [PubMed] [Google Scholar]
- 15.Smaoui S, Elleuch L, Bejar W, Karray-Rebai I, Ayadi I, Jaouadi B, et al. Inhibition of fungi and Gram-negative bacteria by bacteriocin BacTN635 produced by Lactobacillus plantarum sp. TN635. Appl Biochem Biotechnol. 2010;162:1132–46. doi: 10.1007/s12010-009-8821-7. [DOI] [PubMed] [Google Scholar]
- 16.Salaj R, Stofilová J, Soltesová A, Hertelyová Z, Hijová E, Bertková I, et al. The effects of two Lactobacillus plantarum strains on rat lipid metabolism receiving a high fat diet. ScientificWorldJournal. 2013;2013:135142. doi: 10.1155/2013/135142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saavedra L, Taranto MP, Sesma F, de Valdez GF. Homemade traditional cheeses for the isolation of probiotic Enterococcus faecium strains. Int J Food Microbiol. 2003;88:241–5. doi: 10.1016/s0168-1605(03)00186-7. [DOI] [PubMed] [Google Scholar]
- 18.Kumar A, Saini P, Shrivastava JN. Production of peptide antifungal antibiotic and biocontrol activity of Bacillus subtilis. Indian J Exp Biol. 2009;47:57–62. [PubMed] [Google Scholar]
- 19.Sewell RD, Rafieian-Kopaei M. The history and ups and downs of herbal medicine usage. J Herbmed Pharmacol. 2014;3:1–3. [Google Scholar]
- 20.Nasri H, Baradaran A, Shirzad H, Rafieian-Kopaei M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int J Prev Med. 2014;5:1487–99. [PMC free article] [PubMed] [Google Scholar]
- 21.Bahmani M, Shirzad H, Rafieian S, Rafieian-Kopaei M. Silybum marianum: Beyond hepatoprotection. J Evid Based Complementary Altern Med. 2015;20:292–301. doi: 10.1177/2156587215571116. [DOI] [PubMed] [Google Scholar]
- 22.Ebrahimie M, Bahmani M, Shirzad H, Rafieian-Kopaei M, Saki K. A review study on the effect of Iranian herbal medicines on opioid withdrawal syndrome. J Evid Based Complementary Altern Med. 2015;20:302–9. doi: 10.1177/2156587215577896. [DOI] [PubMed] [Google Scholar]
- 23.Asadi-Samani M, Rafieian-Kopaei M, Azimi N. Gundelia: A systematic review of medicinal and molecular perspective. Pak J Biol Sci. 2013;16:1238–47. doi: 10.3923/pjbs.2013.1238.1247. [DOI] [PubMed] [Google Scholar]
- 24.Bahmani M, Banihabib E, Rafieian-Kopaei M, Gholami-Ahangaran M. Comparison of disinfection activities of nicotine with copper sulphate in water containing Limnatis nilotica. Kafkas Univ Vet Fak Derg. 2015;21:9–11. [Google Scholar]
- 25.Nasri H, Behradmanesh S, Ahmadi A, Rafieian-Kopaei M. Impact of oral Vitamin D (cholecalciferol) replacement therapy on blood pressure in type 2 diabetes patients; a randomized, double-blind, placebo controlled clinical trial. J Nephropathol. 2014;3:29–33. doi: 10.12860/jnp.2014.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amini FG, Rafieian-Kopaei M, Nematbakhsh M, Baradaran A, Nasri H. Ameliorative effects of metformin on renal histologic and biochemical alterations of gentamicin-induced renal toxicity in Wistar rats. J Res Med Sci. 2012;17:621–5. [PMC free article] [PubMed] [Google Scholar]
- 27.Nasri H, Mortazavi M, Ghorbani A, Shahbazian H, Kheiri S, Baradaran A, et al. Oxford-MEST classification in IgA nephropathy patients: A report from Iran. J Nephropathol. 2012;1:31–42. doi: 10.5812/jnp.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rafieian-Kopaei M, Asgary S, Adelnia A, Setorki M, Khazaei M, Kazemi S, et al. The effects of cornelian cherry on atherosclerosis and atherogenic factors in hypercholesterolemic rabbits. J Med Plants Res. 2011;5:2670–6. [Google Scholar]
- 29.Aroutcheva AA, Simoes JA, Faro S. Antimicrobial protein produced by vaginal Lactobacillus acidophilus that inhibits Gardnerella vaginalis. Infect Dis Obstet Gynecol. 2001;9:33–9. doi: 10.1155/S1064744901000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boris S, Jiménez-Díaz R, Caso JL, Barbés C. Partial characterization of a bacteriocin produced by Lactobacillus delbrueckii subsp. lactis UO004, an intestinal isolate with probiotic potential. J Appl Microbiol. 2001;91:328–33. doi: 10.1046/j.1365-2672.2001.01403.x. [DOI] [PubMed] [Google Scholar]
- 31.Ogunbanwo ST, Sanni AI, Onilude AA. Characterization of bacteriocin produced by Lactobacillus plantarum F1 and Lactobacillus brevis OG1. Afr J Biotechnol. 2003;2:219–27. [Google Scholar]
- 32.Coconnier MH, Lievin V, Hemery E, Servin AL. Antagonistic activity against Helicobacter infection in vitro and in vivo by the human Lactobacillus acidophilus strain LB. Appl Environ Microbiol. 1998;64:4573–80. doi: 10.1128/aem.64.11.4573-4580.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Millette M, Luquet FM, Lacroix M. In vitro growth control of selected pathogens by Lactobacillus acidophilus- and Lactobacillus casei-fermented milk. Lett Appl Microbiol. 2007;44:314–9. doi: 10.1111/j.1472-765X.2006.02060.x. [DOI] [PubMed] [Google Scholar]
- 34.Chassarard C, Grattepanche F, Lacroix C. Probiotics and health claim: Challenges for tailoring their efficacy. In: Kneifel W, Salminen S, editors. Probiotics and Health Claims. Wiley-Blackwell: John Wiley & Sons;; 2011. pp. 49–74. [Google Scholar]
- 35.Rossetti L, Fornasari ME, Gatti M, Lazzi C, Neviani E, Giraffa G. Grana Padano cheese whey starters: Microbial composition and strain distribution. Int J Food Microbiol. 2008;127:168–71. doi: 10.1016/j.ijfoodmicro.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Schillinger U, Geisen R, Holzapfel WH. Potential of antagonistic microorganisms and bacteriocins for the biological preservation of foods. Trends Food Sci Technol. 1996;7:158–64. [Google Scholar]


