Abstract
Background
The antiviral effect of HBV in different nucleos (t) ide analogues is still not well known. This study was conducted to compare the effectiveness of lamivudine (LMV), adefovir dipivoxil (ADV), telbivudine (LdT), and entecavir (ETV) monotherapy in chronic HBeAg-negative hepatitis B patients with medium load of HBV DNA.
Material/Methods
The effective data of 207 patients treated by LMV (n=43), ADV (n=57), LdT (n=54) or ETV (n=53) were collected and analyzed during 144-week follow-up by retrospective analysis.
Results
Serum HBV DNA levels were significantly lower in the ETV group 1.91±0.45 log10 IU/ml) than in the LdT group (2.09±0.62 log10 IU/ml), ADV group (2.26±0.73 log10 IU/ml), and LMV group (2.08±0.75 log10 IU/ml) at 12 weeks (P=0.0464). HBV DNA levels were maintained at lower levels in the ETV group compared to other 3 groups during follow-up (48 weeks, P<0.001; 96 weeks, P<0.001). Multivariate Cox regression analysis showed that LMV (P=0.001), ADV, (P<0.001), and LdT (P<0.001) were all negative predictors of HBV DNA-negative time, but ETV was not. Viral breakthrough occurred in 34.8% (15/43) of patients in the LMV group; 5.26% (3/57) in the ADV group, 7.4.0% (4/54) in the LdT group, and 0% (0/53) in the ETV group at the end of follow-up. No significant differences were found in mean ALT levels (all P>0.05) or in cumulative normalization rates (P=0.473).
Conclusions
ETV was more potent and faster for viral response and lower viral breakthrough in medium load of HBV DNA when compared to LMV, ADV, or LdT monotherapy in HBeAg-negative CHB.
MeSH Keywords: Hepatitis B; Hepatitis, Chronic; Lamivudine
Background
Hepatitis B is a serious global health issues and about 0.5 to 1.2 million deaths per year are attributed to HBV-associated complications, including cirrhosis, hepatic decompensation, and liver cancer [1–3]. A very important goal of hepatitis B therapy is to reduce HBV DNA replication, which can improve patient survival by preventing disease progression from cirrhosis and hepatocellular carcinoma [4,5].
Lamivudine (LMV), adefovir dipivoxil (ADV), telbivudine (LdT), entecavir (ETV), and tenofovir (TDF) are now available nucleos (t) ide analogues (NAs) for HBV used in China. LMV LdT, and especially ADV are relatively less potent than ETV or tenofovir in suppressing viral replication [6,7]. But it is reported that ADV showed good effectiveness, tolerance, and safety profiles in chronic hepatitis B (CHB) patients with a relatively lower viral load [8]. This is why some patients chose ADV or LMV for antiviral therapy in China. On the other hand, LMV, ADV, and LdT showed a lower resistant barrier than ETV and TDF [9]. More common use of these medicines could increase the resistant risk in long-term therapy. As a result, they were no longer recommended as first-line drugs for anti-HBV therapy in Europe and elsewhere [1,10]. On the other hand, TDF has only been approved in CFDA for less than 1 year, so there is insufficient data on its effects; thus, we did not compare TDF in this research.
As a compromise, all 4 NAs approved are recommended by the Chinese guideline for management of chronic hepatitis B [10]. Actually, many more CHB patients in China, especially in second-tier cities or rural areas, chose LMV, ADV, or LdT than ETV for antiviral therapy.
We have good reason to believe that LMV, ADV, and LdT would show a relatively poorer effectiveness than ETV in CHB patients with a higher viral load, but they might show the same effect while in patients with a medium HBV DNA (the amount of HBV DNA load was between 4–6 log10 IU/ml). It will be very useful in making decision of choosing the NAs when patient with medium HBV DNA load and there will have more choices for antiviral drugs. Because HBeAg-negative CHB had lower HBV DNA levels in most situation, we conduct this study to evaluate the effectiveness in real life of the 4 NAs in relatively lower viral load (HBV DNA 4–5 log10 IU/ml) in HBeAg-negative CHB, which would be good beneficial for most patients in China.
Material and Methods
Study protocol
This study was retrospectively conducted in our follow-up clinic. All patients enrolled in this study were enrolled between January 2012 and December 2015. All of the enrolled patients were managed according to the 2015 Chinese official guidelines for the treatment of CHB [10].
Definition
Medium HBV DNA level were defined as HBV DNA levels in the serum ≥4 log10 IU/ml, but no more than 6 log10 IU/ml; HBV DNA-negative was defined as HBV DNA levels less than the detection limit (100 IU/ml). Complete viral response (CVR) was defined as HBV DNA lower than the detection limit at any time. Viral breakthrough (VB) was defined as an increase in serum HBV DNA by >1 log10 IU/ml above last treatment. The rate of antiviral drugs altered was defined as the need to change antiviral drugs because of resistance, partial virological response, or adverse events. HBeAg serum conversion is defined as change of HBeAg from positive to negative. ALT normalization is defined as ALT level decreased into the normal range (5–35 U/L).
Exclusions
None of the subjects had ever received anti-HBV therapy before. Patients were excluded for: HBV DNA levels that were too low or too high (<4 log10 IU/ml or >6 log10 IU/ml); co-infected with HCV infection and other virus-related hepatitis; autoimmune hepatitis; hepatocellular carcinoma; Wilson’s disease; coexistent with human immunodeficiency infection; hepatotoxicity; steatogenic; antineoplastic; systemic immuno-modulator treatment within a period of 6 months before the start of antiviral therapy; primary biliary cirrhosis, primary sclerosing cholangitis; and a history of psychiatric disease.
Among 463 patients treated with 4 NAs, 255 were excluded because of various reasons (all show in Figure 1). Finally, 207 NAs-naïve patients with CHB were enrolled and treated with LMV (LMV group, n=43), ADV (ADV group, n=57), LdT (LdT group n=54) or ETV (ETV group, n=53) monotherapy. These patients received 100 mg/day LMV (GlaxoSmithKline), 10 mg mg/day ADV (GlaxoSmithKline), 600 mg/day LdT (Novartis), or 0.5 mg/day ETV (Bristol-Myers Squibb,) respectively.
Figure 1.
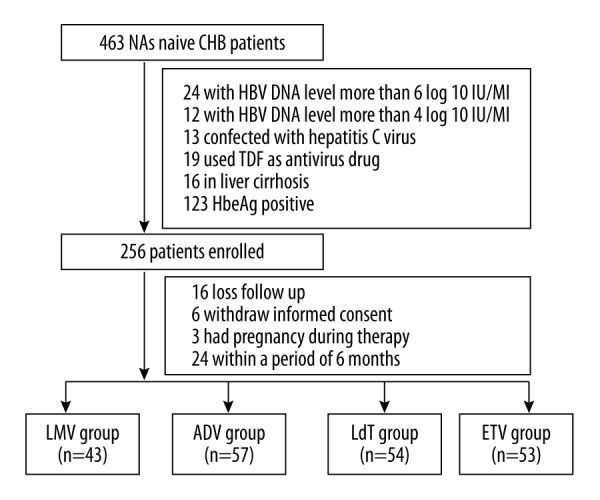
The flow chart of the patients enrolled.
Assessment of clinical and laboratory data
The baseline demographic and serologic data were obtained from the patients; we aimed to find associated factors of the 4 groups before the antivirus treatment. Then the patient biochemical data were monitored every 3 months, including HBV DNA level, hepatis surface antigen (HBsAg), Hepatitis B e antigen (HBeAg), anti-HBe HBeAg, ALT, aspartate aminotransferase (AST), total bilirubin (TB), and creatinine (Cr) until the end of follow-up.
HBV DNA was quantified by real-time PCR (Da a Genetics, lower limits of detection: 100IU/ml). HBsAg, anti-HBs, HBeAg, and anti-HBe were measured using commercially available chemiluminescence assay kits (Roche Diagnostic Systems).
Ethics statement
The study was approved by the Third Affiliated Hospital Ethics Committee of Sun Yat-sen University and adhered to the guidelines of the Declaration of Helsinki. The study design and manuscript preparation fully followed the guidelines from the STROBE statement [11]. Informed consent was obtained from each enrolled patient.
Statistical analysis
All data were entered and analyzed by using the SPSS software vision 20.0 (SPSS 20.0 Inc., USA). Categorical data were presented as counts and percentages and were analyzed by using the chi-Square test or the Fisher exact test. Continuous quantitative data are showed as mean ± standard deviation (SD) and were analyzed by Student’s t test. Cox regression analysis was performed in search of variables determining the virological response. Cumulative rates of complete viral suppression were analyzed by the Kaplan-Meier method. P<0.05 was considered statistically significant. All statistical tests were two-tailed and p-values less than 0.05 were considered as statistically significant.
Results
Subject disposition
A total of 207 patients were enrolled in this retrospective study. Baseline demographic and disease characteristics including serum ALT levels and HBV DNA levels were comparable in the 4 treatment groups (Table 1). The mean HBV DNA level was 4.93±0.73 log10 IU/ml. In all, 43 (20.7%), 57 (27.5%), 54 (26.1%), and 53 (25.6%) patients were in the LMV, ADV, LdT, and ETV groups, respectively. All NAs-naïve patients of CHB remained for the duration of all antiviral treatment during 144 weeks.
Table 1.
Baseline of patients with HBeAg negative CHB patients in LMV, ADV, LdT, ETV patients respectively.
| Baseline | LMV (n=43) | ADV (n=57) | LDT (n=54) | ETV (n=53) | P |
|---|---|---|---|---|---|
| Age (years) | 40.0±8.7 | 40.1±11.9 | 40.4±11.6 | 44.7±9.1 | 0.0697 |
| Sex (Male, n, %) | 35 (81.4) | 45 (78.9) | 41 (75.9) | 48 (87.3) | 0.484 |
| Body mass Index (kg/m2) | 20.7±1.9 | 21.0±2.0 | 20.7±1.5 | 20.6±1.6 | 0.772 |
| The proportion of alcohol history (%) | 18.6 (8/43) | 29.8 (17/57) | 16.7 (9/54) | 30.2 (16/53) | 0.219 |
| The proportion of smoking history (%) | 32.6 (14/43) | 22.8 (13/57) | 31.5 (17/54) | 22.6 (12/53) | 0.524 |
| Family history of HBV, n (%) | 48.8 (21/43) | 31.6 (18/57) | 22.2 (12/54) | 17.0 (9/53) | 0.04 |
| ALT, U/L | 103.7±51.6 | 109.5±93.5 | 138.0±128.9 | 126.6±83.2 | 0.251 |
| HBV DNA,log10 IU/ml | 4.92±0.74 | 4.78±0.79 | 4.93±0.76 | 5.10±0.60 | 0.1464 |
Virologic response
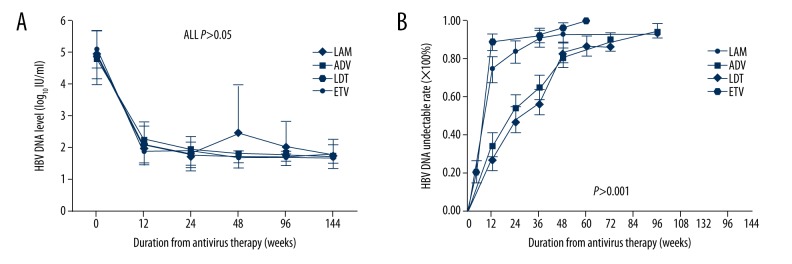
Serum HBV DNA levels were significantly lower in the ETV group (1.91±0.45 log10 IU/ml) than in the LdT group (2.09±0.62 log10 IU/ml), ADV group (2.26±0.73 log10 IU/ml), and LMV group (2.08±0.75 log10 IU/ml) at 12 weeks (P=0.0464), and the HBV DNA levels were still lower in the ETV group compared to the other 3 groups during follow-up (48 weeks, P<0.001; 96 weeks, P<0.001) (Table 2). The HBV DNA levels in the LMV group were slightly increased from week 48 to 96 because of many viral breakthroughs, but other groups did not show the same trend (Figure 2A). HBV DNA undetectable rates were increased in all 4 groups; the ETV group increased faster than in the LMV, ADV, and LdT groups (P<0.001), and ADV and LdT were increased nearly the same as each other (Figure 2B). The median survival time of HBV DNA undetectable limits of corresponding survival analysis in LMV, ADV, LdT, and ETV groups were 12, 24, 36, and 12 weeks, respectively (P<0.001). Multivariate Cox regression analysis showed that compared to the ETV group, LMV (P=0.001, OR [odds ratio]=0.454), ADV (P<0.001, OR=0.367), and LdT (P<0.001, OR=0.381) all were negative predictors in HBV DNA-negative time (Table 3).
Table 2.
HBV DNA levels in different time points during the whole follow up periods in four groups patients.
| HBV DNA (log10 IU/ml) | LAM (n=43) | ADV (n=57) | LdT (n=54) | ETV (n=53) | P |
|---|---|---|---|---|---|
| Baseline | 4.92±0.74 | 4.78±0.79 | 4.93±0.76 | 5.10±0.60 | 0.146 |
| 12 week | 2.08±0.75 | 2.26±0.73 | 2.09±0.62 | 1.91±0.45 | 0.0464 |
| 24 week | 1.80±0.38 | 1.93±0.63 | 1.79±0.39 | 1.91±0.45 | 0.3459 |
| 48 week | 2.48±1.52 | 1.82±0.45 | 1.73±0.19 | 1.70±0.01 | <0.001 |
| 96 week | 2.03±0.82 | 1.80±0.36 | 1.73±0.15 | 1.70±0.11 | <0.001 |
| 144 week | 1.79±0.29 | 1.75±0.22 | 1.81±0.46 | 1.70±0.02 | 0.292 |
Figure 2.
The dynamic changes in HBV DNA levels and HBV DNA undetectable rates. HBV DNA levels (A) were decreased significantly at week 12 and maintain at low levels after week 12 in four group patients, while LMV group were increased slightly at week 48 because of viral breakthrough. Cumulative incidence of patients with undetectable serum HBV DNA rates (B) were also increased significantly at week 12 in four groups patients, especially in ETV group.
Table 3.
Multivariate Cox regression analysis in HBV DNA negative time.
| B | SE | Wald | df | P | Exp (B) | 95.0% CI for Exp (B) | ||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Age | .005 | .007 | .598 | 1 | .439 | 1.006 | .992 | 1.020 |
| Sex | .251 | .195 | 1.666 | 1 | .197 | 1.286 | .878 | 1.883 |
| BMI | .069 | .045 | 2.342 | 1 | .126 | 1.072 | .981 | 1.171 |
| Smoking history | .034 | .182 | .036 | 1 | .850 | 1.035 | .725 | 1.478 |
| Family history of of hepatitis B | .060 | .158 | .142 | 1 | .706 | 1.062 | .778 | 1.448 |
| Alcohol history | .054 | .218 | .061 | 1 | .805 | 1.055 | .688 | 1.619 |
| ETV | 25.755 | 3 | .000 | |||||
| LAM | −.790 | .229 | 11.926 | 1 | .001 | .454 | .290 | .711 |
| ADV | −.978 | .222 | 19.401 | 1 | .000 | .376 | .243 | .581 |
| LdT | −.965 | .219 | 19.504 | 1 | .000 | .381 | .248 | .585 |
| HBV DNA baseline level | −.016 | .109 | .021 | 1 | .884 | .984 | .795 | 1.219 |
| ALT baseline level | .000 | .001 | .027 | 1 | .869 | 1.000 | .998 | 1.001 |
Virological breakthrough
Viral breakthrough occurred in 34.8% (5/43) in the LMV group, 5.26% (3/57) in the ADV group, 7.4.0% (4/54) in the LdT group, and 0% (0/53) in ETV group at the end of follow-up (P<0.001). Twelve patients in the LMV group with viral breakthrough had genotypic resistance (all were rtM204I/V) at weeks 48 to 72, and 3 patients did not. All the patients with viral breakthrough had ADV added at weeks 60 to 72 and they had good viral response in followed-up. Two patients in the ADV group who had viral breakthrough had genotypic resistance (one was rtA181V at week 96 and the other was rtN236T at week 120) and they were switched to TDF; the other patient did not have any genotypic resistance. All 4 patients in the LdT group with viral breakthrough had rtM204I/V at weeks 60 to 96 and they were put on ADV to get a viral response after another 12-week follow-up.
Biochemical response
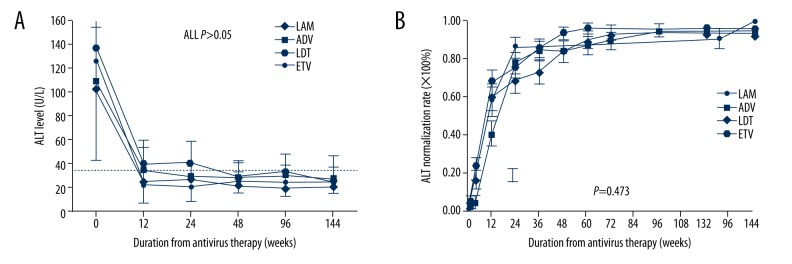
Serum ALT levels decreased progressively during 144 weeks of treatment in the 4 patient groups, as shown in Figure 3A. Throughout the treatment period, the mean serum ALT levels declined significantly at 12 weeks in all 4 group patients compared to the beginning of antiviral therapy (all P<0.001), and there was no significant difference from week 12 to 144 (all, P>0.05). The cumulative ALT normalization rates increased after antiviral periods and no significant differences were observed in the 4 groups (P=0.473, Figure 3B)
Figure 3.
The mean ALT levels and cumulative ALT normalization rates during antiviral treatment in four groups patients. The mean ALT levels (A) were declined significant to normal ranges from week 12 to the end of follow up. There was no significant difference in any time point of the whole follow up periods. Cumulative incidence of patients of ALT normalization (B) showed that ALT normalization rates were all increased in four groups and no significant differences were found either.
Safety
During antiviral therapy, no patients died or needed to receive liver transplantation. LdT was switched to LMV in 2 patients (2/54, 3.70%) due to increased serum creatine kinase, but recovered after 3 months. The only adverse effect found was in 3 patients (3/57, 5.26%) in the ADV group who had increased serum creatine. Although LMV had no drug-related adverse events, 15 patients in the lamivudine group (15/43, 34.8%) had to add ADV to the treatment regimen because of viral breakthrough or poor viral response during follow-up. No patients had adverse events and none needed to change regimen in the ETV group patients
Discussion
Nowadays in China, LMV is still widely used as monotherapy for CHB. Many patients with low viral load also choose ADV as antiviral drugs. ETV is superior to LMV in terms of virologic, biochemical, and histologic outcomes in NA-naive CHB patients, and is also superior in economic analysis [12,13]. However, the efficacy of antiviral drugs such as LMV, ADV, LdT, and ETV in the medium HBV DNA level in HBeAg-negative CHB patients is still unknown. In this study, we showed that ETV has better virologic response than LMV, ADV, and LdT in NA-naïve Chinese patients with medium HBV DNA levels in HBeAg-negative CHB during the 144-week treatment process.
HBV DNA levels in the 4 groups of CHB patients declined significantly, especially in the ETV groups. Multivariate Cox regression analysis of HBV DNA-negative time also showed that LMV, LdT, and ADV were all negative predictors compared to ETV. These results suggest the potent antiviral capacity of ETV from commencement of antiviral therapy up to 12 weeks. However, with the extension of antiviral time after 12 weeks, the 4 NAs had similar inhibition of HBV DNA replication if there was no poor response or drug resistance. Regarding HBV DNA undetectable rates, ETV was significantly higher than the other NAs at 12 weeks. These results were similar to those of Lampertico in a study of ETV monotherapy in 418 NA-naïve CHB patients, in which the HBV DNA undetectable rates increased progressively from 68% at 6 months to 85% at 1 year [14]. Therefore, ETV has earlier and faster inhibition effects on HBV DNA replication compared to other NAs.
The mean ALT levels declined significantly at the earlier stage, were maintained at near normal range in all groups, and there were no significant differences among different groups. The ALT normalization rates also showed the quick biomedical response after antiviral treatment, although no significant differences were found in the 4 groups. These results indicate that for HBeAg-negative CHB patients with medium HBV DNA levels, there was no significant difference in the ALT normalization rates after antiviral treatment.
Viral breakthrough in ETV was very low in many clinical studies. Du et al. reported only 1 case in 50 patients in the ETV group who had no response during 104-week follow-up in a recent study [15], and no ETV drug resistance was found in our study. The rates of viral breakthrough in ETV were much lower than in other groups, and complete viral response in the ETV was highest. The rates of antiviral drugs altered in the ETV group was also lower than in other groups. These results suggest that ETV is a faster and more potent antiviral drug in HBV suppression.
Because of its retrospective design, there may be some limitations in our study. Firstly, the sample size was small and follow-up time was short. Secondly, most results in this study were in agreement with other research around the world. However, we are not sure that they were done by head-to-head researches for 4 drugs and in the baseline for medium HBV DNA levels in China. Nevertheless, the patients were followed-up in our clinic for a long time and we had good compliance; otherwise, they would be excluded from follow-up. Therefore, this study is meaningful and trustworthy, and provides better suggestions of drug selections in China.
Conclusions
In conclusion, even in the medium load of NA-naïve CHB patients, ETV was more potent and faster for viral response and lower viral breakthrough in medium load of HBV DNA when compared to LMV, ADV, or LdT monotherapy in HBeAg-negative CHB, while it no advantage in ALT normalization rates.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest related to the publication of this manuscript.
Source of support: This project was supported by Guangdong Science and Technology Plan Projects (NO. 2014A020212478)
References
- 1.European Association for The Study of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57( 1):167–85. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Rapti I, Hadziyannis S. Risk for hepatocellular carcinoma in the course of chronic hepatitis B virus infection and the protective effect of therapy with nucleos (t) ide analogues. World J Hepatol. 2015;7( 8):1064–73. doi: 10.4254/wjh.v7.i8.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baran B. Nucleos (t) ide analogs in the prevention of hepatitis B virus related hepatocellular carcinoma. World J Hepatol. 2015;7( 13):1742–54. doi: 10.4254/wjh.v7.i13.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol Int. 2016;10( 1):1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 6.Liu F, Wang X, Wei F, et al. Efficacy and resistance in de novo combination lamivudine and adefovir dipivoxil therapy versus entecavir monotherapy for the treatment-naive patients with chronic hepatitis B: A meta-analysis. Virol J. 2014;11:59. doi: 10.1186/1743-422X-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buti M, Tsai N, Petersen J, et al. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig Dis Sci. 2015;60( 5):1457–64. doi: 10.1007/s10620-014-3486-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segovia MC, Chacra W, Gordon SC. Adefovir dipivoxil in chronic hepatitis B: History and current uses. Expert Opin Pharmacother. 2012;13( 2):245–54. doi: 10.1517/14656566.2012.649727. [DOI] [PubMed] [Google Scholar]
- 9.Tacke F, Kroy DC. Treatment for hepatitis B in patients with drug resistance. Ann Transl Med. 2016;4( 18):334. doi: 10.21037/atm.2016.09.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chinese Society of Hepatology, Chinese Medical Association; Chinese Society of Infectious Diseases, Chinese Medical Association. Hou JL, Lai W. [The guideline of prevention and treatment for chronic hepatitis B: A 2015 update]. Zhonghua Gan Zang Bing Za Zhi. 2015;23( 12):888–905. doi: 10.3760/cma.j.issn.1007-3418.2015.12.002. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 11.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007;370:1453–57. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 12.Lee KK, Wu DB, Chow PY, et al. Economic analysis between entecavir and lamivudine for the treatment of chronic hepatitis B in Hong Kong. J Gastroenterol Hepatol. 2012;27( 7):1167–74. doi: 10.1111/j.1440-1746.2011.07047.x. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Huo M, Chao J, Liu P. Application of bayesian approach to cost-effectiveness analysis of antiviral treatments in chronic hepatitis B. PLoS One. 2016;11( 8):e0161936. doi: 10.1371/journal.pone.0161936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lampertico P, Vigano M, Soffredini R, et al. Entecavir monotherapy for nuc-naive chronic hepatitis B patients from field practice: High efficacy and favorable safety profile over 3 years. Hepatology. 2011;54(Suppl 1) Abstract 1436. [Google Scholar]
- 15.Du QW, Ding JG, Sun QF, et al. Combination lamivudine and adefovir versus entecavir for the treatment of naïve chronic hepatitis B patients: A pilot study. Med Sci Monit. 2013;19:751–56. doi: 10.12659/MSM.889443. [DOI] [PMC free article] [PubMed] [Google Scholar]