ABSTRACT
Many intracellular pathogens survive and replicate within vacuole-like structures in the cytoplasm. It has been unclear how the host immune system controls such pathogen-containing vacuoles. Interferon-inducible GTPases are dynamin-like GTPases that target the membranes of pathogen-containing vacuoles. Upon their oligomerization on the membrane, the vacuole structure disintegrates and the pathogen gets exposed to the hostile cytoplasm. What has been obscure is how the immune system detects and directs the GTPases to these pathogen shelters. Using a common protist parasite of mice, Toxoplasma gondii, we found that the LC3 conjugation system of autophagy is necessary and sufficient for targeting the interferon-inducible GTPases to membranes. We dubbed this process Targeting by AutophaGy proteins (TAG). In canonical autophagy, the LC3 conjugation system is required to form membrane-bound autophagosomes, which encircle and deliver cytosolic materials to lysosomes for degradation. In TAG, however, the conjugation system is required to mark the membranes of pathogen-containing vacuoles with ubiquitin-like LC3 homologs, which function as molecular beacons to recruit the GTPases to their target membranes. Our data suggest that the LC3 conjugation system of autophagy plays an essential role in detecting and marking pathogen-containing vacuoles for immune effector targeting by the host immune system.
KEYWORDS: autophagy, GBP, IFN, IRG, LC3, TAG, targeting, Toxoplasma gondii, ubiquitin
Enemy in disguise: How are pathogen-containing vacuoles targeted?
Many intracellular pathogens survive and replicate within vacuole-like structures, which are usually made by pathogens through reorganization of existing cellular membrane structures.1 This so-called pathogen-containing vacuole provides pathogens not only a shelter from the host immune defense system but also a base to exploit the host cells. Thus, for the fitness and survival of the host, the operating base of pathogens has to be detected and destroyed by the immune defense system.2 The immune system may have evolved to recognize these abnormally reorganized membrane structures as pathogen-associated molecular patterns, yet it has been obscure whether such pattern recognition receptor exists against pathogen-containing vacuoles.2
What has been known are effector proteins used by the host immune system to fight these vacuolar pathogens; immunity related GTPases (IRGs) and guanylate-binding proteins (GBPs) are interferon-inducible, dynamin-like GTPases that destroy these pathogen shelters.3,4 Upon their induction, preferentially by interferon-gamma (IFNG), these effectors rapidly accumulate on the membranes of pathogen-containing vacuoles. The targeted membranes are subsequently vesiculated and eventually rupture, exposing the resident pathogens to the host cytoplasm. The consequences of such exposure include inhibition of the pathogen replication, activation of cytosolic pathogen sensors, and subsequent death of the pathogens and/or the host cells.5-7 In contrast to these outcomes, it has been poorly understood how the IRGs and GBPs are directed to the membrane of these vacuoles.8
To explain the targeting mechanism of the IRGs and GBPs, a prevalent model in the field has been the “guard” model (also similarly known as “missing-self” model).2,9,10 The gist of the “guard” model is a set of “guard” proteins that mark and protect the host cell membranes from the “executer” IFN-inducible GTPases. The IRG family is subdivided into GMS IRGs and GKS IRGs, based on their sequence in a conserved GTPase domain.11 Membrane-bound GMS IRGs (e.g. IRGM1, IRGM2, and IRGM3/IGTP) are considered the “guard” proteins and predominantly cytosolic GKS IRGs (e.g., IRGA6/IIGP1 and IRGB6/TGTP1) function as the “executer” proteins: the “guard” GMS IRGs act as guanosine nucleotide dissociation inhibitor (GDI) of “executer” GKS IRG proteins, keeping the GKS IRGs in their inactive GDP-bound form.9,11 In the case of Toxoplasma gondii, the parasitophorus vacuole membrane (PVM) of T. gondii is derived from the host plasma membrane while T. gondii removes most (if not all) of the membrane-associated host proteins.12 This suggests that the PVM of T. gondii may not be protected with the “guard” proteins and thus may be identified as a target membrane for the “executer” proteins. The GKS IRGs can translocate to the PVM by simple diffusion and can be activated to bind GTP in the absence of the “guard.” Subsequently, their GTP-dependent oligomerization may lead to the vesiculation and consequent disruption of the parasitophorus vacuole of T. gondii.13-16 Further, the IRG system has been shown to control the localization of GBPs onto the PVM through ubiquitination, although the mechanism is still not completely understood.17-19 Therefore, the “guard” model predicted that any endomembrane structure without the protective “guard” can be targeted spontaneously by GKS IRGs and subsequently GBPs.2
Intriguingly, several groups found that Atg5, an essential autophagy gene, is required to target these GKS IRGs and GBPs to the membranes of vacuoles containing pathogens like Chlamydia trachomatis and T. gondii.10,15,20,21 Without Atg5, GKS IRGs and GBPs are induced normally by IFNG, but they form aggregates in the cytoplasm rather than targeting pathogen-containing vacuoles.22 Since the major function of autophagy is to deliver cytoplasmic materials to lysosomes for degradation23 and the cytoplasmic aggregates of GKS IRGs in Atg5 knockout cells were composed of GTP-bound active forms,20,24 it was proposed that the degradative autophagy pathway might be required to maintain a functional pool of the IFN-inducible GTPases by removing falsely aggregated GTPases.20
Targeting by AutophaGy proteins (TAG): LC3 homologs mark membranes to be targeted
Using a well-established murine model of protist T. gondii infection, we examined the role of the autophagy pathway in proper targeting of IFN-inducible GTPases to the membranes of pathogen-containing vacuoles. Contrary to the expected, lysosomal degradation through autophagy did not affect targeting of GKS IRGs and GBPs to the PVM of T. gondii and subsequent control of T. gondii replication by IFNG.25 Pharmacological induction or inhibition of the autophagy pathway also did not play any role in the targeting process. Further, genetic ablation of other essential autophagy genes (e.g., Ulk1, Ulk2, Atg14) had no effect on targeting of IFN-inducible GTPases. These data clearly demonstrated that the targeting process is independent of the degradative autophagy pathway but dependent on Atg5.25
Atg5 is an essential gene for the formation of double-membrane-bound autophagosomes, which sequester and transport cytosolic materials to lysosomes.26 Autophagosome formation requires the conjugation of ubiquitin-like microtubule-associated-protein-1-light-chain-3 (LC3) and its homologs to phosphatidylethanolamine (PE) on membranes, which is essential for the extension of the membrane and the completion of the globular autophagosome. For the conjugation of LC3 homologs, ATG5 forms a protein complex with ATG12 and ATG16L1, and they function as an E3-like ligase complex with an E1-like activating enzyme, ATG7, and an E2-like conjugating enzyme, ATG3.23,27 We found that not only ATG5 but the entire LC3 conjugation system (ATG7, ATG3, and ATG12–ATG5-ATG16L1 complex) of autophagy is necessary to target LC3, GKS IRGs and GBPs to the PVM of T. gondii and subsequent control of T. gondii infection in vitro and in vivo by IFNG,25 which is consistent with recent findings from other groups.21,28 Collectively, we found that the targeting process of GKS IRGs and GBPs is governed by a non-canonical and non-degradative function of the LC3 conjugation system of autophagy.25
Since the only known function of the entire LC3 conjugation system is indeed to conjugate LC3 homologs to a membrane,29 we further examined whether the conjugation of LC3 homologs is required for the targeting process. Multiple LC3 homologs exist in mammalian systems, and they act in different stages of autophagosome formation.30 The LC3 subfamily (LC3A and LC3B in mice) functions in elongation of the autophagosomal membrane and the GABARAP subfamily (GABARAP, GABARAPL1, and GABARAPL2) works in a later stage of autophagosome completion.31 Recent studies further established the difference between these 2 subfamilies with respect to their interaction partners.32-35 In spite of these distinct autophagic functions of LC3 homologs, we found that all LC3 homologs play an essential but overlapping function for targeting of the GKS IRGs and GBPs to the PVM and subsequent control of T. gondii infection by IFNG.36 That is, either LC3 or GABARAP subfamily alone was sufficient for proper targeting of GKS IRGs and GBPs, and only in the absence of both subfamilies the targeting process was disrupted. Our data suggest that both subfamilies of LC3 homologs function analogously in recruiting the IFN-inducible GTPases to the PVM of T. gondii.36 The 2 subfamilies share a ubiquitin-like core domain but possess dissimilar N-termini, which are known to be essential for their distinct autophagic functions.30 Thus, their overlapping function in TAG suggest that the shared ubiquitin-like domain may play a crucial role in the targeting process, while the difference at the N-termini may be removed by a potential post-translational modification during the process.
A crucial question was whether the LC3 conjugation system is not only necessary but also sufficient for targeting the GKS IRGs and GBPs to a membrane. That is, can the LC3 conjugation system define the targeting site of GKS IRGs and GBPs? Since the E3-like ATG12–ATG5-ATG16L1 complex specifies the conjugation site of LC3 homologs,29 to examine this possibility, we relocated the ATG12–ATG5-ATG16L1 complex to plasma membrane or mitochondria outer membrane, using the KRAS-CAAX motif and a modified anchor-away system, respectively.29,37 In these settings, the IFN-inducible GTPases relocated to the plasma membrane and mitochondria outer membrane, where the LC3 and the conjugation system relocated.36 These data clearly showed that the LC3 conjugation system indeed can specify the target membrane of the GKS IRGs and GBPs and further suggest that LC3 homologs on the PVM of T. gondii are the factors that recruit the IFN-inducible GTPases specifically to the target membrane.
How do LC3 homologs recruit the IFN-inducible GTPases to the target membrane?
Since LC3 localizes on the outer (cytosolic side) membrane of cellular autophagosome,38 in theory LC3-decorated autophagosomes would be targeted and disrupted by the GKS IRGs and GBPs upon their induction by IFN, if LC3 on the membrane is the only necessary signal to recruit them. In fact, IRGs have been shown to interact with autophagy proteins and to be involved in canonical degradative autophagy.39-42 In these reports, however, IRGs stimulate autophagy rather than interfere, and furthermore we have not observed any significant effect of IFNG on canonical degradative autophagy.43 Although we cannot exclude the possibility that the special nature of the autophagosome (e.g. double-membrane) prevents it from being disrupted by GKS IRGs and GBPs upon their targeting, similar to the lysosome,44 our data suggest that the IFN-inducible GTPases do not target and affect the autophagosomes decorated with LC3.
One relevant observation to these outstanding questions is that T. gondii infection differentially affected the recruitment of the GKS IRGs and GBPs to the membrane where LC3 localized.36 In the absence of T. gondii infection, induction of GKS IRGs and GBPs by IFNG was not sufficient to send them noticeably to the mitochondria outer membrane marked with LC3 via the anchor-away system. In contrast, upon T. gondii infection of the IFNG-treated cells, both GTPases were substantially recruited to the LC3-marked mitochondria membrane.36 Intriguingly, the recruitment of GKS IRGs to the plasma membrane marked with LC3 via the KRAS-CAAX system was not dependent on T. gondii infection.36 In this regard, it is interesting to note that there is no known GMS IRGs on the plasma membrane45 whereas IRGM1 localizes on mitochondria.46 Thus, T. gondii infection may affect the recruitment of, at least, GKS IRGs to the LC3-marked membranes by altering the localization of GMS IRGs. Further, we also observed that stably expressed IFN-inducible GTPase did not go to the PVM of T. gondii unless the infected cells were activated with IFNG (unpublished). It is also noteworthy that the localization of LC3 homologs on the PVM of T. gondii was not dependent on IFNG signal but was substantially enhanced upon IFNG treatment,36 indicating a potential role of IFNG in modifying the function of the LC3 conjugation system and/or the LC3 homologs on the membrane. Taken together, these data suggest that cellular events induced by IFNG and T. gondii infection may substantially modify the interaction between LC3 homologs and the IFN-inducible GTPases.
Both IFNG treatment and T. gondii infection can induce substantial changes of gene expressions and signaling pathways in target cells.47-49 Further, LC3 homologs can be post-translationally modified in various ways.50-53 Therefore, it is tempting to speculate a ‘triple-check’ model of IFN, LC3, and infection to explain how LC3 homologs recruit the IFN-inducible GTPases to the target membrane: we hypothesize that IFN enables LC3 homologs on a membrane to function as ‘guanine nucleotide exchange factor (GEF)’ for the local activation of the IFN-inducible GTPases and T. gondii infection causes the LC3 homolog-marked membranes to be free of GMS IRGs that function as ‘GDI’ for the inactivation of the GTPases.9 Such ‘IFN-activation’ of LC3 homologs may work as direct post-translational modifications of the LC3 homologs or through targeting of additional factors (e.g., ubiquitin18,19) that can work with the LC3 homologs. As a necessary corollary, the GKS IRGs and GBPs would be activated and multimerize on a membrane with GEF, ‘IFN-activated’ LC3 homologs, and without GDI, GMS IRGs.
This model predicts that expression of GKS IRGs or GBPs,44 especially their activated forms,54 over a control capacity of GMS IRGs may simply override such ‘triple-check’ restriction. Likewise, this ‘triple-check’ model can also explain a previous finding on the recruitment of GKS IRGs and GBPs to lipid droplet (LD) in the absence of T. gondii infection.44,54 In wild type cells, LDs are marked with IRGM1, IRGM3 and some LC3.54 However, in the absence of Irgm1 and Irgm3, LC3 accumulates substantially on LDs and the GKS IRGs and GBPs are targeted to the LDs.54 Since GMS IRGs are not on the LDs, ‘IFN-activated’ LC3 on the LD might be sufficient to recruit the GKS IRGs and GBPs in the absence of T. gondii infection, just like the LC3-marked plasma membrane.36
For the case of autophagosomes, we speculate that an IFN-mediated change of LC3 may not occur due to the potential inaccessibility of LC3 upon its occupation by other autophagic process related proteins38 or due to some other special nature of the autophagosome.55 In this regard, it is noteworthy that the LC3 on autophagosomes is partially removed by the deconjugating enzyme ATG4 via some incompletely understood mechanism,56,57 suggesting a limited accessibility of the LC3 on autophagosome for modification. Since the membrane of autophagosomes is derived from various sources of endomembranes,58 autophagosomal membranes are likely to be associated with GMS IRGs.44 Further, both mouse IRGM1 and its human homolog IRGM interact with many key autophagy proteins, and at least partial localization of them on autophagosomes were reported.41,59,60 Thus, autophagosomes may be heavily associated with GMS IRGs to the extent which T. gondii infection may not considerably alter GMS localization. Further studies will illuminate the functional mechanism of the TAG process, including this ‘triple-check’ model of IFN, LC3, and infection.
Does the E3-like ATG12–ATG5-ATG16L1 complex detect pathogen-containing vacuoles?
If the LC3 homologs can specify where the GKS IRGs and GBPs go and the E3-like ATG12–ATG5-ATG16L1 complex determines where the LC3 homologs are conjugated to, then what brings the ATG12–ATG5-ATG16L1 complex to the membrane of pathogen-containing vacuoles? In fact, we were able to detect the complex on the PVM of T. gondii in as early as 2 minutes-post-infection of T. gondii,36 which is similar kinetics to the initiation of canonical autophagy.61 Such swift recruitment suggests that detection of T. gondii invasion occurs quickly without transcriptional or translational change in the infected cells. In theory, the ATG12–ATG5-ATG16L1 complex may go to the target membrane, directly by recognizing the abnormally reorganized membrane structure of T. gondii PV or indirectly by another upstream sensor that recognizes the structure as a pathogen-associated molecular pattern.
ATG5 can bind membranes without ATG12 and ATG16L1.62 Recent data from the yeast ATG12–ATG5-ATG16 complex further showed that the direct membrane binding activity of ATG5 is inhibited by ATG12 conjugation and the inhibition is relieved upon its binding to ATG16.63 Thus, the ATG12–ATG5-ATG16 complex can directly bind to membranes through ATG5, yet the complex doesn't significantly associate with membranes in vivo without pro-autophagic stimulus.64 These data suggest that the direct membrane binding activity of ATG5 in the complex is further restricted by currently unknown factors. Thus, we speculate that the absence of an unknown inhibitory signal on the PVM of T. gondii, at least transiently, may lead to the recruitment of the ATG12–ATG5-ATG16L1 complex to the membrane via ATG5, as proposed in the “guard” model or “missing-self” model.2,9,10
Alternatively, but not exclusively, the complex may be recruited to the PVM of T. gondii via an interaction partner of ATG16L1 on the membrane. In canonical autophagy, the ATG12–ATG5-ATG16L1 complex is recruited to the site of autophagosome initiation via WIPI2b (WD repeat domain, phosphoinositide interacting 2b). WIPI2b can bind to phosphatidylinositol 3-phosphate (PtdIns3P) at the initiation site and bring the complex to the site via its interaction with the coiled-coil domain of ATG16L1.65 The coiled-coil domain of ATG16L1 is essential for autophagosome formation in vivo, through oligomerization of ATG16L1 and interaction with upstream autophagy genes.64-67 Intriguingly, the coiled-coil domain of ATG16L1 is also required for the IFNG-mediated control of T. gondii infection,36 although we did not observe any significant role of PtdIns3P in the control of T. gondii by IFNG.25 These data may suggest that targeting process require the oligomerization of ATG16L1, but the coiled-coil domain of ATG16L1 may be required to bring the ATG12–ATG5-ATG16L1 complex through its interaction with a potential sensor on the PVM of T. gondii. In this regard, it is worth noting that the PVM of T. gondii was rapidly marked with GFP fused to the pleckstrin homology domain of AKT, which recognizes a membrane containing PtdIns(3,4,5)P3 or PtdIns(3,4)P2.49 Taken together, the ATG12–ATG5-ATG16L1 complex may be recruited to the PVM of T. gondii via another protein that can bind both the phosphorylated derivatives of phosphatidylinositol on the PVM and ATG16L1, in a similar fashion to the initiation of canonical autophagosome formation.
A current working model for the TAG-mediated control of T. gondii infection
We found that the LC3 conjugation system of autophagy marks the membrane of pathogen-containing vacuole to be targeted and disrupted by the IFN-inducible GTPases. Importantly, IFN is not required for the LC3 conjugation system to mark the membrane;36 that is, with or without the activation of cells with IFN, pathogen-containing vacuoles get marked with the LC3 homologs. However, we also observed substantially enhanced localization of LC3 homologs on the PVM of T. gondii upon IFNG treatment.36 Only upon activation of cells with IFN, the GKS IRGs and GBPs are induced and then targeted to the LC3-marked membranes for their effector function. Based on our data, we propose the following working model for the TAG-mediated control of T. gondii infection (Fig. 1): 1) upon invasion and formation of the PV of T. gondii, the ATG12–ATG5-ATG16L1 complex is recruited to the PVM, 2) the complex conjugates the LC3 homologs on the PVM of T. gondii and dissociates, 3) the conjugated LC3 homologs on the PVM are ‘IFN-activated’ and recruit induced GKS IRGs and GBPs upon the activation of cells with IFNG, 4) GKS IRGs and GBPs on the PVM get activated in the absence of GMS IRGs and disrupt the membrane by vesiculation, and 5) T. gondii exposed to cytoplasm upon the PV disintegration is killed and further activates the immune system. There are many remaining questions to be answered in order to understand the TAG of IFN-inducible GTPases. How the ATG12–ATG5-ATG16L1 complex is involved in sensing the invasion of vacuolar pathogens and how the LC3 homologs bring the GKS IRGs and GBPs specifically to the target membrane will be the next key questions to be tackled.
Figure 1.
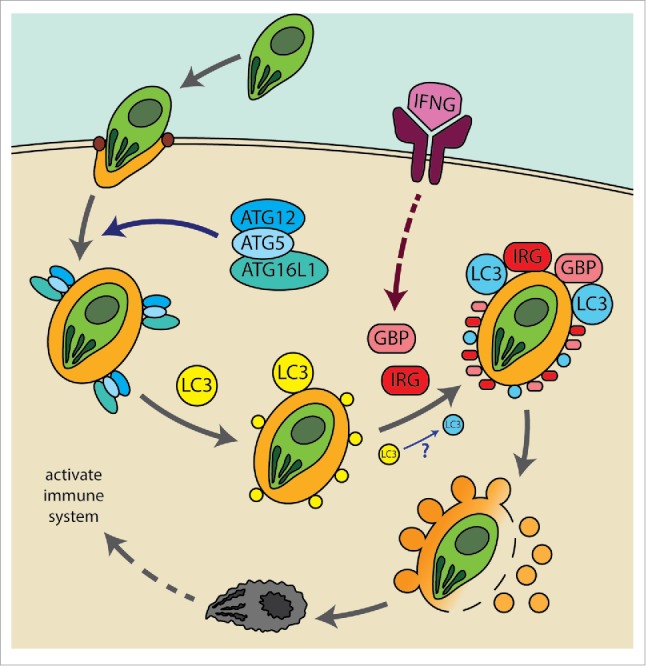
A current working model for the TAG-mediated control of T. gondii infection. Upon invasion and formation of the PV of T. gondii, the ATG12–ATG5-ATG16L1 complex is recruited to the PVM. The complex conjugates the LC3 homologs on the PVM of T. gondii, and the conjugated LC3 homologs on the PVM are activated by IFNG (e.g. post-translational modification of the LC3 homologs or through targeting of additional factors [e.g., ubiquitin18,19] that can work with the LC3 homologs) and then recruit the GKS IRGs (for simplicity, just indicated as IRG in the figure) and GBPs upon their induction by IFNG. GKS IRGs and GBPs on the PVM disrupt the membrane by vesiculation, and T. gondii exposed to cytoplasm upon the PV disintegration gets killed and further activates the immune system.
The host immune system has evolved a defense strategy to sense and attack abnormally reorganized endomembrane structures as pathogen-associated molecular patterns. Understanding this immune defense strategy of the host and potential evasion strategies of pathogens would allow us to develop more effective therapeutics against the diseases caused by vacuolar pathogens.
Abbreviations
- ATG
autophagy related
- GABARAP
GABA type A receptor-associated protein
- GBP
guanylate binding protein
- IFN
interferon
- IGTP
interferon gamma induced GTPase
- IIGP1
interferon inducible GTPase 1
- IRG
immunity-related GTPase
- LC3
microtubule associated protein 1 light chain 3
- PE
phosphatidylethanolamine
- PVM
parasitophorous vacuole membrane
- TAG
targeting by autophagy proteins
- TGTP1
T cell specific GTPase 1
- ULK
uncoordinated 51-like kinase
- WIPI2b
WD repeat domain, phosphoinositide interacting 2b.
funding
This work was supported by startup fund to S.H., and in part by Brinson Foundation Junior Investigator Grant, Cancer Research Foundation, an Institutional Research Grant (IRG-58-004-53-IRG) from the American Cancer Society, the University of Chicago Comprehensive Cancer Center Support Grant (P30 CA14599), the University of Chicago Digestive Diseases Research Core Center (NIDDK P30DK42086), and Cancer Center Core facilities (DNA sequencing and genotyping, flow cytometry, integrated microscopy, and monoclonal antibody). S.B.B. was supported by Molecular and Cellular Biology Training Grant (T32 GM007183).
References
- [1].Randow F, MacMicking JD, James LC. Cellular self-defense: how cell-autonomous immunity protects against pathogens. Science 2013; 340:701-6; PMID:23661752; https://doi.org/ 10.1126/science.1233028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Coers J. Self and non-self discrimination of intracellular membranes by the innate immune system. PLoS Pathog 2013; 9:e1003538; PMID:24068918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim B-H, Shenoy AR, Kumar P, Bradfield CJ, MacMicking JD. IFN-Inducible GTPases in Host Cell Defense. Cell Host Microbe 2012; 12:432-44; PMID:23084913; https://doi.org/ 10.1016/j.chom.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Pilla-Moffett D, Barber MF, Taylor GA, Coers J. Interferon-Inducible GTPases in Host Resistance, Inflammation and Disease. Journal of Molecular Biology 2016; PMID:27181197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hunn JP, Feng CG, Sher A, Howard JC. The immunity-related GTPases in mammals: a fast-evolving cell-autonomous resistance system against intracellular pathogens. Mamm Genome 2010; 22:43-54; PMID:21052678; https://doi.org/ 10.1007/s00335-010-9293-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kim BH, Shenoy AR, Kumar P, Das R, Tiwari S, MacMicking JD. A family of IFN- -inducible 65-kD GTPases protects against bacterial infection. Science 2011; 332:717-21; PMID:21551061; https://doi.org/ 10.1126/science.1201711 [DOI] [PubMed] [Google Scholar]
- [7].Meunier E, Dick MS, Dreier RF, Schürmann N, Broz DK, Kenzelmann Broz D, Warming S, Roose-Girma M, Bumann D, Kayagaki N, et al.. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature 2014; 509:366-70; PMID:24739961; https://doi.org/ 10.1038/nature13157 [DOI] [PubMed] [Google Scholar]
- [8].Meunier E, Broz P. Interferon-inducible GTPases in cell autonomous and innate immunity. Cell Microbiol 2016; 18:168-180; PMID:26572694; https://doi.org/ 10.1111/cmi.12546 [DOI] [PubMed] [Google Scholar]
- [9].Hunn JP, Koenen-Waisman S, Papic N, Schroeder N, Pawlowski N, Lange R, Kaiser F, Zerrahn J, Martens S, Howard JC. Regulatory interactions between IRG resistance GTPases in the cellular response to Toxoplasma gondii. EMBO J 2008; 27:2495-509; PMID:18772884; https://doi.org/ 10.1038/emboj.2008.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Haldar AK, Saka HA, Piro AS, Dunn JD, Henry SC, Taylor GA, Frickel EM, Valdivia RH, Coers J. IRG and GBP host resistance factors target aberrant, “non-self” vacuoles characterized by the missing of ‘self’ IRGM proteins. PLoS Pathog 2013; 9:e1003414; PMID:23785284; https://doi.org/ 10.1371/journal.ppat.1003414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].MacMicking JD. Immune control of phagosomal bacteria by p47 GTPases. Curr Opin Microbiol 2005; 8:74-82; PMID:15694860; https://doi.org/ 10.1016/j.mib.2004.12.012 [DOI] [PubMed] [Google Scholar]
- [12].Sinai AP. Biogenesis of and activities at the Toxoplasma gondii parasitophorous vacuole membrane. Subcell Biochem 2008; 47:155-64; PMID:18512349; https://doi.org/ 10.1007/978-0-387-78267-6_12 [DOI] [PubMed] [Google Scholar]
- [13].Martens S, Parvanova I, Zerrahn J, Griffiths G, Schell G, Reichmann G, Howard JC. Disruption of Toxoplasma gondii Parasitophorous Vacuoles by the Mouse p47-Resistance GTPases. PLoS Pathog 2005; 1:e24; PMID:16304607; https://doi.org/ 10.1371/journal.ppat.0010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, Ferguson DJP, Yap GS. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med 2006; 203:2063-71; PMID:16940170; https://doi.org/ 10.1084/jem.20061318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhao YO, Khaminets A, Hunn JP, Howard JC. Disruption of the toxoplasma gondii parasitophorous vacuole by IFNγ-inducible immunity-related GTPases (IRG Proteins) triggers necrotic cell death. PLoS Pathog 2009; 5:e1000288; PMID:19197351; https://doi.org/ 10.1371/journal.ppat.1000288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Khaminets A, Hunn JP, Könen-Waisman S, Zhao YO, Preukschat D, Coers J, Boyle JP, Ong Y-C, Boothroyd JC, Reichmann G, et al.. Coordinated loading of IRG resistance GTPases on to the Toxoplasma gondii parasitophorous vacuole. Cell Microbiol 2010; 12:939-61; PMID:20109161; https://doi.org/ 10.1111/j.1462-5822.2010.01443.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Traver MK, Henry SC, Cantillana V, Oliver T, Hunn JP, Howard JC, Beer S, Pfeffer K, Coers J, Taylor GA. Immunity-related GTPase M (IRGM) proteins influence the localization of guanylate-binding protein 2 (GBP2) by modulating macroautophagy. J Biol Chem 2011; 286:30471-80; PMID:21757726; https://doi.org/ 10.1074/jbc.M111.251967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee Y, Sasai M, Ma JS, Sakaguchi N, Ohshima J, Bando H, Saitoh T, Akira S, Yamamoto M. p62 Plays a specific role in interferon-γ-induced presentation of a toxoplasma vacuolar antigen. Cell Rep 2015; 13:223-233; PMID:26440898; https://doi.org/ 10.1016/j.celrep.2015.09.005 [DOI] [PubMed] [Google Scholar]
- [19].Haldar AK, Foltz C, Finethy R, Piro AS, Feeley EM, Pilla-Moffett DM, Komatsu M, Frickel E-M, Coers J. Ubiquitin systems mark pathogen-containing vacuoles as targets for host defense by guanylate binding proteins. Proc Natl Acad Sci USA 2015; 112:E5628-37; PMID:26417105; https://doi.org/ 10.1073/pnas.1515966112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Selleck EM, Fentress SJ, Beatty WL, Degrandi D, Pfeffer K, Virgin HW, MacMicking JD, Sibley LD. Guanylate-binding Protein 1 (Gbp1) Contributes to Cell-autonomous Immunity against Toxoplasma gondii. PLoS Pathog 2013; 9:e1003320; PMID:23633952; https://doi.org/ 10.1371/journal.ppat.1003320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Haldar AK, Piro AS, Pilla DM, Yamamoto M, Coers J. The E2-like conjugation enzyme Atg3 promotes binding of IRG and Gbp proteins to chlamydia- and toxoplasma-containing vacuoles and host resistance. PLoS One 2014; 9:e86684; PMID:24466199; https://doi.org/ 10.1371/journal.pone.0086684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zhao Z, Fux B, Goodwin M, Dunay IR, Strong D, Miller BC, Cadwell K, Delgado MA, Ponpuak M, Green KG, et al.. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe 2008; 4:458-69; PMID:18996346; https://doi.org/ 10.1016/j.chom.2008.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature 2011; 469:323-35; PMID:21248839; https://doi.org/ 10.1038/nature09782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Papic N, Hunn JP, Pawlowski N, Zerrahn J, Howard JC. Inactive and Active States of the Interferon-inducible Resistance GTPase, Irga6, in Vivo. J Biol Chem 2008; 283:32143-51; PMID:18784077; https://doi.org/ 10.1074/jbc.M804846200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Choi J, Park S, Biering SB, Selleck E, Liu CY, Zhang X, Fujita N, Saitoh T, Akira S, Yoshimori T, et al.. The parasitophorous vacuole membrane of Toxoplasma gondii is targeted for disruption by ubiquitin-like conjugation systems of autophagy. Immunity 2014; 40:924-35; PMID:24931121; https://doi.org/ 10.1016/j.immuni.2014.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov 2012; 11:709-30; PMID:22935804; https://doi.org/ 10.1038/nrd3802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rubinsztein DC, Shpilka T, Elazar Z. Mechanisms of Autophagosome Biogenesis. Current Biology 2012; 22:R29-R34; PMID:22240478; https://doi.org/ 10.1016/j.cub.2011.11.034 [DOI] [PubMed] [Google Scholar]
- [28].Ohshima J, Lee Y, Sasai M, Saitoh T, Su Ma J, Kamiyama N, Matsuura Y, Pann-Ghill S, Hayashi M, Ebisu S, et al.. Role of mouse and human autophagy proteins in IFN-γ-induced cell-autonomous responses against Toxoplasma gondii. J Immunol 2014; 192:3328-35; PMID:24563254; https://doi.org/ 10.4049/jimmunol.1302822 [DOI] [PubMed] [Google Scholar]
- [29].Fujita N, Itoh T, Omori H, Fukuda M, Noda T, Yoshimori T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol Biol Cell 2008; 19:2092-100; PMID:18321988; https://doi.org/ 10.1091/mbc.E07-12-1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shpilka T, Weidberg H, Pietrokovski S, Elazar Z. Atg8: an autophagy-related ubiquitin-like protein family. Genome Biol 2011; 12:226; PMID:21867568; https://doi.org/ 10.1186/gb-2011-12-7-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Weidberg H, Shvets E, Shpilka T, Shimron F, Shinder V, Elazar Z. LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J 2010; 29:1792-802; PMID:20418806; https://doi.org/ 10.1038/emboj.2010.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Engedal N, Seglen PO. Autophagy of cytoplasmic bulk cargo does not require LC3. Autophagy 2016; 12:439-41; PMID:26237084; https://doi.org/ 10.1080/15548627.2015.1076606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lystad AH, Ichimura Y, Takagi K, Yang Y, Pankiv S, Kanegae Y, Kageyama S, Suzuki M, Saito I, Mizushima T, et al.. Structural determinants in GABARAP required for the selective binding and recruitment of ALFY to LC3B-positive structures. EMBO Rep 2014; 15:557-65; PMID:24668264; https://doi.org/ 10.1002/embr.201338003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Olsvik HL, Lamark T, Takagi K, Larsen KB, Evjen G, Øvervatn A, Mizushima T, Johansen T. FYCO1 contains a C-terminally extended, LC3A/B-preferring LC3-interacting Region (LIR) motif required for efficient maturation of autophagosomes during basal autophagy. J Biol Chem 2015; 290:29361-74; PMID:26468287; https://doi.org/ 10.1074/jbc.M115.686915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Szalai P, Hagen LK, Sætre F, Luhr M, Sponheim M, Øverbye A, Mills IG, Seglen PO, Engedal N. Autophagic bulk sequestration of cytosolic cargo is independent of LC3, but requires GABARAPs. Exp Cell Res 2015; 333:21-38; PMID:25684710; https://doi.org/ 10.1016/j.yexcr.2015.02.003 [DOI] [PubMed] [Google Scholar]
- [36].Park S, Choi J, Biering SB, Dominici E, Williams LE. Targeting by AutophaGy proteins (TAG): Targeting of IFNG-inducible GTPases to membranes by the LC3 conjugation system of autophagy. Autophagy 2016; 12:1153-67; PMID:27172324; https://doi.org/ 10.1080/15548627.2016.1178447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Haruki H, Nishikawa J, Laemmli UK. The Anchor-away technique: rapid, conditional establishment of yeast mutant phenotypes. Mol Cell 2008; 31:925-32; PMID:18922474; https://doi.org/ 10.1016/j.molcel.2008.07.020 [DOI] [PubMed] [Google Scholar]
- [38].Fu M-M, Nirschl JJ, Holzbaur ELF. LC3 Binding to the Scaffolding protein JIP1 regulates processive dynein-driven transport of autophagosomes. Dev Cell 2014; 29:577-90; PMID:24914561; https://doi.org/ 10.1016/j.devcel.2014.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Singh SB, Davis AS, Taylor GA, Deretic V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science 2006; 313:1438-41; PMID:16888103; https://doi.org/ 10.1126/science.1129577 [DOI] [PubMed] [Google Scholar]
- [40].He S, Wang C, Dong H, Xia F, Zhou H, Jiang X, Pei C, Ren H, Li H, Li R, et al.. Immune-related GTPase M (IRGM1) regulates neuronal autophagy in a mouse model of stroke. Autophagy 2012; 8:1621-7; PMID:22874556; https://doi.org/ 10.4161/auto.21561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chauhan S, Mandell MA, Deretic V. IRGM governs the core autophagy machinery to conduct antimicrobial defense. Mol Cell 2015; 58:507-21; PMID:25891078; https://doi.org/ 10.1016/j.molcel.2015.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Al-Zeer MA, Al-Younes HM, Braun PR, Zerrahn J, Meyer TF. IFN-γ-Inducible Irga6 mediates host resistance against chlamydia trachomatis via autophagy. PLoS ONE 2009; 4:e4588; PMID:19242543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hwang S, Maloney NS, Bruinsma MW, Goel G, Duan E, Zhang L, Shrestha B, Diamond MS, Dani A, Sosnovtsev SV, et al.. Nondegradative role of Atg5-Atg12/ Atg16L1 autophagy protein complex in antiviral activity of interferon gamma. Cell Host Microbe 2012; 11:397-409; PMID:22520467; https://doi.org/ 10.1016/j.chom.2012.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Maric-Biresev J, Hunn JP, Krut O, Helms JB, Martens S, Howard JC. Loss of the interferon-γ-inducible regulatory immunity-related GTPase (IRG), Irgm1, causes activation of effector IRG proteins on lysosomes, damaging lysosomal function and predicting the dramatic susceptibility of Irgm1-deficient mice to infection. BMC Biology 2016; 14:1-20; PMID:26728391; https://doi.org/ 10.1186/s12915-016-0255-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].da Fonseca Ferreira-da-Silva M, Springer-Frauenhoff HM, Bohne W, Howard JC. Identification of the Microsporidian Encephalitozoon cuniculi as a New Target of the IFNγ-Inducible IRG Resistance System. PLoS Pathog 2014; 10:e1004449; PMID:25356593; https://doi.org/ 10.1371/journal.ppat.1004449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Springer HM, Schramm M, Taylor GA, Howard JC. Irgm1 (LRG-47), a regulator of cell-autonomous immunity, does not localize to mycobacterial or listerial phagosomes in IFN-γ-induced mouse cells. J Immunol 2013; 191:1765-1774; PMID:23842753; https://doi.org/ 10.4049/jimmunol.1300641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Blader IJ, Manger ID, Boothroyd JC. Microarray analysis reveals previously unknown changes in Toxoplasma gondii-infected human cells. J Biol Chem 2001; 276:24223-31; PMID:11294868; https://doi.org/ 10.1074/jbc.M100951200 [DOI] [PubMed] [Google Scholar]
- [48].Yarovinsky F. Innate immunity to Toxoplasma gondiiinfection. Nat Rev Immunol 2014; 14:109-21; PMID:24457485; https://doi.org/ 10.1038/nri3598 [DOI] [PubMed] [Google Scholar]
- [49].Muniz-Feliciano L, Van Grol J, Portillo J-AC, Liew L, Liu B, Carlin CR, Carruthers VB, Matthews S, Subauste CS. Toxoplasma gondii-Induced Activation of EGFR Prevents Autophagy Protein-Mediated Killing of the Parasite. PLoS Pathog 2013; 9:e1003809; PMID:24367261; https://doi.org/ 10.1371/journal.ppat.1003809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Huang R, Xu Y, Wan W, Shou X, Qian J, You Z, Liu B, Chang C, Zhou T, Lippincott-Schwartz J, et al.. Deacetylation of nuclear LC3 drives autophagy initiation under starvation. Mol Cell 2015; 57:456-66; PMID:25601754; https://doi.org/ 10.1016/j.molcel.2014.12.013 [DOI] [PubMed] [Google Scholar]
- [51].He H. Post-translational modifications of three members of the human MAP1LC3 family and detection of a novel type of modification for MAP1LC3B. J Biol Chem 2003; 278:29278-87; PMID:12740394; https://doi.org/ 10.1074/jbc.M303800200 [DOI] [PubMed] [Google Scholar]
- [52].Xie Y, Kang R, Sun X, Zhong M, Huang J, Klionsky DJ, Tang D. Posttranslational modification of autophagy-related proteins in macroautophagy. Autophagy 2015; 11:28-45; PMID:25484070; https://doi.org/ 10.4161/15548627.2014.984267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Wilkinson DS, Jariwala JS, Anderson E, Mitra K, Meisenhelder J, Chang JT, Ideker T, Hunter T, Nizet V, Dillin A, et al.. Phosphorylation of LC3 by the Hippo Kinases STK3/STK4 Is Essential for Autophagy. Mol Cell 2015; 57:55-68; PMID:25544559; https://doi.org/ 10.1016/j.molcel.2014.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Haldar AK, Saka HA, Piro AS, Dunn JD, Henry SC, Taylor GA, Frickel EM, Valdivia RH, Coers J. IRG and GBP host resistance factors target aberrant, “non-self” vacuoles characterized by the missing of ‘self’ IRGM proteins. PLoS Pathog 2013; 9:e1003414-6; PMID:23785284; https://doi.org/ 10.1371/journal.ppat.1003414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kubota C, Torii S, Hou N, Saito N, Yoshimoto Y, Imai H, Takeuchi T. Constitutive reactive oxygen species generation from autophagosome/lysosome in neuronal oxidative toxicity. J Biol Chem 2010; 285:667-74; PMID:19850931; https://doi.org/ 10.1074/jbc.M109.053058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yu Z-Q, Ni T, Hong B, Wang H-Y, Jiang F-J, Zou S, Chen Y, Zheng X-L, Klionsky DJ, Liang Y, et al.. Dual roles of Atg8−PE deconjugation by Atg4 in autophagy. Autophagy 2014; 8:883-92; PMID:NOT_FOUND; https://doi.org/ 10.4161/auto.19652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Fernández AF, Lopez-Otin C. The functional and pathologic relevance of autophagy proteases. J Clin Invest 2015; 125:33-41; PMID:Can't; https://doi.org/ 10.1172/JCI73940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Bento CF, Renna M, Ghislat G, Puri C, Ashkenazi A, Vicinanza M, Menzies FM, Rubinsztein DC. Mammalian Autophagy: How Does It Work? Annu Rev Biochem 2016; 85:685-713; PMID:26865532; https://doi.org/ 10.1146/annurev-biochem-060815-014556 [DOI] [PubMed] [Google Scholar]
- [59].Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 2004; 119:753-66; PMID:15607973; https://doi.org/ 10.1016/j.cell.2004.11.038 [DOI] [PubMed] [Google Scholar]
- [60].GrEgoire IP, Richetta C, Meyniel-Schicklin L, Borel S, Pradezynski F, Diaz O, Deloire A, Azocar O, Baguet J, Le Breton M, et al.. IRGM Is a Common Target of RNA Viruses that Subvert the Autophagy Network. PLoS Pathog 2011; 7:e1002422; PMID:22174682; https://doi.org/ 10.1371/journal.ppat.1002422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Koyama-Honda I, Itakura E, Fujiwara TK, Mizushima N. Temporal analysis of recruitment of mammalian ATG proteins to the autophagosome formation site. Autophagy 2013; 9:1491-1499; PMID:23884233; https://doi.org/ 10.4161/auto.25529 [DOI] [PubMed] [Google Scholar]
- [62].Mizushima N, Yamamoto A, Hatano M, Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y, Yoshimori T. Dissection of autophagosome formation using Apg5-deficient mouse embryonic stem cells. J Cell Biol 2001; 152:657-68; PMID:11266458; https://doi.org/ 10.1083/jcb.152.4.657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Romanov J, Walczak M, Ibiricu I, Schüchner S, Ogris E, Kraft C, Martens S. Mechanism and functions of membrane binding by the Atg5-Atg12/Atg16 complex during autophagosome formation. EMBO J 2012; 31:4304-17; PMID:23064152; https://doi.org/ 10.1038/emboj.2012.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kuma A, Mizushima N, Ishihara N, Ohsumi Y. Formation of the approximately 350-kDa Apg12-Apg5.Apg16 multimeric complex, mediated by Apg16 oligomerization, is essential for autophagy in yeast. J Biol Chem 2002; 277:18619-25; PMID:11897782; https://doi.org/ 10.1074/jbc.M111889200 [DOI] [PubMed] [Google Scholar]
- [65].Dooley HC, Razi M, Polson HEJ, Girardin SE, Wilson MI, Tooze SA. WIPI2 links LC3-conjugation with PI3P, autophagosome formation and pathogen clearance by recruiting Atg12–5-16L1. Mol Cell 2014; 55:238-52; PMID:24954904; https://doi.org/ 10.1016/j.molcel.2014.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Fujioka Y, Noda NN, Nakatogawa H, Ohsumi Y, Inagaki F. Dimeric coiled-coil structure of saccharomyces cerevisiae Atg16 and its functional significance in autophagy. J Biol Chem 2010; 285:1508-15; PMID:19889643; https://doi.org/ 10.1074/jbc.M109.053520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Fujita N, Morita E, Itoh T, Tanaka A, Nakaoka M, Osada Y, Umemoto T, Saitoh T, Nakatogawa H, Kobayashi S, et al.. Recruitment of the autophagic machinery to endosomes during infection is mediated by ubiquitin. J Cell Biol 2013; 203:115-28; PMID:24100292; https://doi.org/ 10.1083/jcb.201304188 [DOI] [PMC free article] [PubMed] [Google Scholar]


