Abstract
This pilot study assessed wireless capsule endoscopy in horses. Image transmission was achieved with good image quality. Time to exit the stomach was variable and identified as one limitation, together with gaps in image transmission, capsule tumbling, and inability to accurately locate the capsule. Findings demonstrate usefulness and current limitations.
Résumé
Existe-t-il une application pour l’endoscopie par capsule sans fil chez les chevaux? Cette étude pilote a évalué l’endoscopie par capsule chez les chevaux. La transmission d’images a permis d’obtenir une bonne qualité d’image. Le temps jusqu’à la sortie de l’estomac était variable et identifié comme une limitation, de même que les lacunes dans la transmission de l’image, le culbutage de la capsule et l’incapacité de situer l’emplacement exact de la capsule. Les résultats démontrent l’utilité et les limitations actuelles.
(Traduit par Isabelle Vallières)
Wireless video capsule endoscopy is a diagnostic imaging technology routinely used in humans (1–3) for noninvasive direct assessment of the small intestine for various disorders, including inflammatory bowel disease (e.g., Crohn’s disease) and gastrointestinal bleeding. More recently, wireless capsule endoscopy has received attention in small animal veterinary medicine (4–6). There is 1 published report of its experimental use in horses (7). Available endoscopy equipment allows for examination of the equine stomach, using a wired 3-meter endoscope (gastroscope). In smaller horses this endoscope can, at best, be advanced to the pylorus and proximal duodenum. Routine direct assessment of most of the small intestine is not possible in the standing, awake horse. The purpose of this prospective pilot study was to test a commercially available human wireless capsule endoscopy system in horses. Our objectives were to: i) determine ease of capsule administration and ability to receive images transmitted by the capsule after administration; ii) assess image quality; iii) determine limitations of using a human capsule in horses; and iv) develop algorithms for computerized image analysis.
Three healthy adult horses (1 Thoroughbred, 2 Quarter Horses) were used in this prospective pilot study. Horses used for this study were ages 4, 10, and 19 y with a mean weight of 507.7 kg (range: 468 to 575 kg). All animal procedures were approved by the University of Saskatchewan Animal Care and Use Committee.
Prior to administration of the capsule, horses were fasted for 24 h, during which they had access to water. The endoscopy capsule (PillCam SB2 and SB3; Medtronic, Brampton, Ontario) SB2 was used for horse 1 and SB3 for horses 2 and 3 (Figure 1a), was administered into the horses’ stomach through a nasogastric tube under standing sedation with xylazine (Rompun 100 mg/mL; Bayer, Toronto, Ontario), 0.5 mg/kg body weight (BW). After administration of the capsule a dosing syringe was used to flush the tube with water. The data recorder was attached behind the shoulder of each horse with use of a surcingle (Figure 1f ). In horse 1, the sensor pads (PillCam SB2, 8 total) were attached to the lateral body wall with Tensoplast® (BSN Medical, Pinetown, 3610, South Africa), 10 cm × 4.5 m; 4 sensors on each side. The hair was clipped in the area of the sensors. In horses 2 and 3 a newer sensor belt system was used and similarly attached. The belt was fastened around the abdomen of the horse midway between the thoracic and the pelvic limbs.
Figure 1.
a — PillCam SB3 Capsule; b — Data recorder with PillCam SB2 sensor pads; c — Data recorder with PillCam SB3 sensor belt; d — Data analyzed on a computer and viewed on RAPID software; e — General setup showing the placement of the wireless capsule endoscopy system components (data recorder and sensors) on the horse; f — Experimental setup in horse 1.
Horses were placed in a stall and constantly monitored for the duration of the 8-hour trial. Two hours after administration of the capsule the horses received free choice hay in a hay net. In each horse, the trial was continued for 8 h, the average battery life of the endoscopy capsule.
Among the capsules that are commercially available, PillCam SB capsules are mainly developed for use in the small bowel. A typical PillCam SB3 capsule (Figure 1a) consists of an 11 × 26 mm dome integrated with a camera, illumination optics, transmitter, and batteries. The capsule sends continuous images acquired during its life to a data recorder through a sensing system consisting of sensor pads (Figure 1b) or a belt (Figure 1c). Finally, the data recorder is connected to a workstation where RAPID software (PillCam; Medtronic) is available for further analysis (Figure 1d). The setup of the data recorder with sensor arrays around the horse is shown in Figures 1e and 1f.
A typical endoscopy capsule when administered to humans works for 8 to 10 h, generating ~ 57 600 frames (8). Evaluating each frame individually would be tiresome and inefficient for a clinician. An automated detection algorithm may be able to mark suspected frames with abnormalities, allowing the clinician to evaluate only the suspected frames to make diagnostic decisions. Such an automated process is not intended to replace the role of the clinician. For the initial development of the algorithm to aid in computerized image analysis in future studies, equine gastroscopy images from clinical cases that have undergone wired gastroscopy were used. Several features of the images were used to classify the frames according to abnormalities (i.e., acute or chronic ulcers) as the images show different properties for different types of abnormalities. These properties were used as features of the classification procedure. The images were first labeled and cropped according to normal and ulcerous regions (Figure 2f ). Each cropped image was read as RGB (Red, Green, Blue) and converted into HSV (Hue, Saturation, Value) plane. In both RGB and HSV planes, statistical features such as mean, standard deviation, kurtosis, skew, and entropy were assessed. Similar features were chosen in a recently published study (9) to detect bleeding in human endoscopy; these worked well. Initial development of the image analysis algorithm showed that not all of the features were significant to classify the abnormalities properly. The training accuracy values of the classifiers were in a range of 85% to 100%.
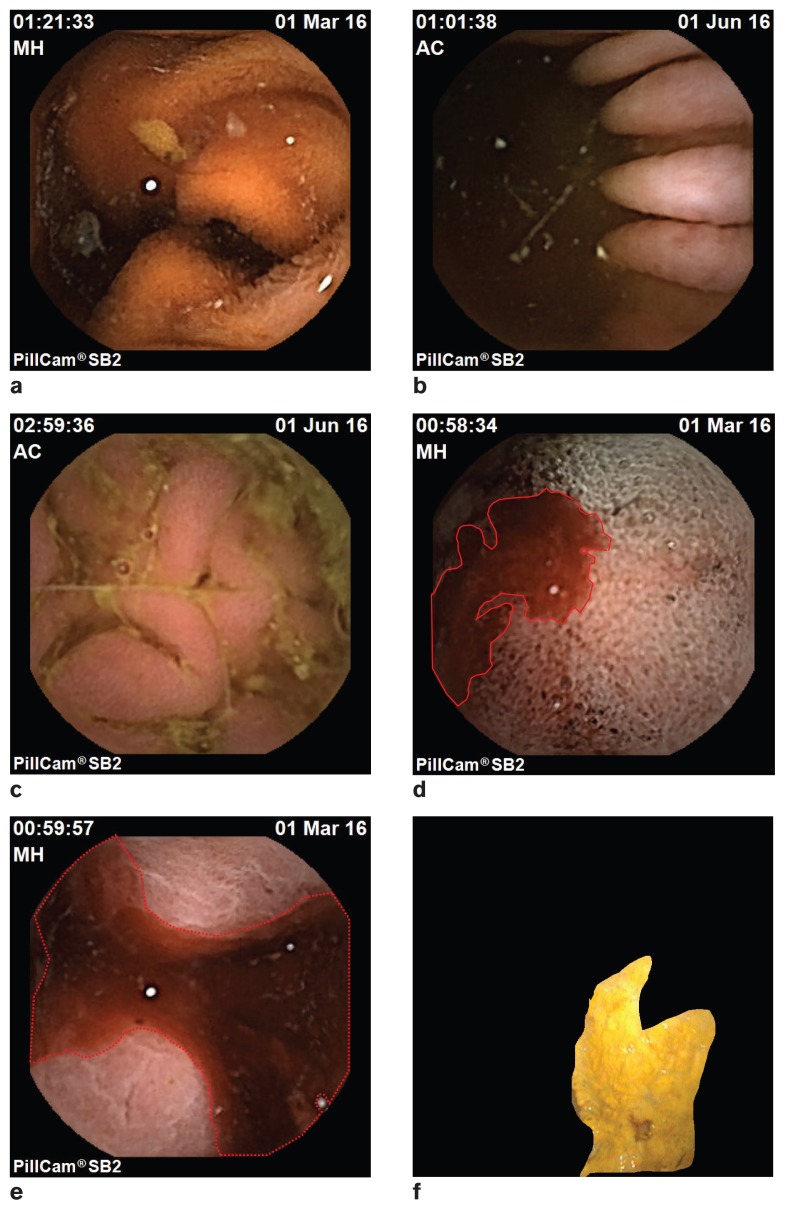
Figure 2.
a to c — Images from the PillCam at various regions of the stomach and small intestine. d to e — PillCam image indicating presence of blood (marked using an auto-detection algorithm during offline diagnosis); f — Regular gastroscopy image with an ulcerous region marked (using automated segmentation algorithm during offline diagnosis).
No complications were observed during or after administration of the capsule. After administration, the endoscopy capsule transmitted real-time images from the gastrointestinal tract of all 3 horses. In horse 1, the capsule entered the small intestine approximately 1 h and 11 min after administration and continued to transmit images until it reached the cecum. Gaps in image transmission were noted when the external sensors could not locate the capsule signal. The total recorded time was 8 h and 23 min, which confirms that the capsule was active for the full duration of its battery life, although significant gaps in image transmission were found. Here, “gap” means loss of communication. Depending on the position of the capsule inside the horse and the positions of the sensors on the belt, some loss of communication was observed. As a result, there were time gaps in the frames received. On an average, the total time-gap was found to be 67 min (131 min for horse 1, 45 min for horse 2, and 27 min for horse 3). It was further noted that some of the images were retrograde as a result of capsule tumbling within the small intestine. Quality of the acquired images was good (Figures 2a, b, c). Here, “good” means “acceptable quality for clinical diagnosis.” The images that were received had clear view of the intestine with good focus and lighting. Very few images were distorted (such as, missing pixels, freezing). Presence of blood (Figures 2d, e) was noted in the stomach of horse 1, which we believe was due to nasal bleeding caused during insertion of the capsule through the nasogastric tube.
The belt system used in horses 2 and 3 resulted in more consistent image transmission. However, the image transmission depends not only the belt but also on the location at which it is positioned and on the size of the horse. It is also to be noted that the capsule took longer to exit the stomach in horse 2 and did not leave the stomach in horse 3 for the duration of the study. The capsules acquired images for 8 h and for 6 h for horses 2 and 3, respectively. There were some missed signals during the experiment, which resulted in loss of frames and therefore, may have resulted in loss of important information as well.
This project was a joint effort between specialists in veterinary medicine and electrical and computer engineering. Results from our pilot study showed that a commercially available endoscopy capsule intended for use in humans (PillCam, Medtronic) was relatively easy to administer into the stomach of horses. When using the sensor pads, there were some gaps in image transmission, but image quality was good, allowing for identification of the section of gastrointestinal tract from which the image was acquired, as well as assessment of the gastrointestinal mucosa. The newer sensor belt technology allowed for almost continuous image transmission in 2 horses, and should be used over the older sensor pad system in future studies.
The main limitations identified included the inability to control the time at which the capsule exits the stomach and the occurrence of capsule tumbling in the small intestine, resulting in acquisition of some retrograde images. Horses were fasted to allow for visualization of the small intestinal mucosa, but this has the disadvantage that fasting reduces gastrointestinal motility. Fasting is required to enable visualization of the small intestinal mucosa; however, the impact of fasting on intestinal motility may be a limitation of wireless capsule endoscopy. Another limitation of the current technology is that the exact location of the endoscopy capsule within the small intestine cannot be determined. This means that even if an abnormality is detected, its exact location would not be known. Tumbling was observed because the horse’s small intestine has a wider diameter than the human small intestine. The small intestinal diameter is what prevents tumbling of the capsule in human patients.
The average recording time of the PillCam (Medtronic) capsule is 8 h. Since it is tiresome and inefficient for a physician or veterinarian to analyze all images that have been acquired we developed an algorithm for automatic detection of abnormalities in endoscopic images. Although PillCam (Medtronic) is able to provide us with a frame rate of 2 to 6 frames/second (fps), a higher frame rate of 10 fps is desired in order to detect the region of interest. Furthermore, the image resolution needs to be enhanced as the diagnostic ability using the algorithm is still error-prone due to the low resolution of the captured images. Using a higher number of image features will make the classification easier. However, a large number of features will make the classifier system bulky. For this reason, the system should be optimized using most significant features only.
Based on the findings from our pilot study, proposed modifications for use of endoscopy capsules in horses include a camera on each end of the capsule to correct for capsule tumbling within the small intestine and the ability to switch on capsule transmission once the capsule exits the stomach in order to save battery life while the capsule is in the stomach. Reduction in capsule size would enable the use of nasogastric tubes with a smaller diameter and therefore allow for use in smaller horses and possibly foals. In order to use wireless capsule endoscopy for routine diagnostic purposes in horses, the ability to accurately locate the position of the capsule within the small intestine would also be required.
A customized equine wireless endoscopy capsule would have several potential uses both for diagnostic and research purposes. It would enable assessment of normal and abnormal small intestinal motility and transit time, aid in the diagnosis of small intestinal problems such as inflammatory bowel disease, neoplasia or post-operative assessment of small intestinal anastomosis and would provide a platform for newer, minimally invasive, diagnostic tools such as optical biopsy. In addition to cameras, the capsule could be equipped with different biochemical sensors that detect specific biomarkers within the small intestine and transmit the data in real time. Examples of biomarkers include inflammatory mediators, products of intestinal metabolism, and analysis of microbial communities.
In conclusion, this initial trial of wireless capsule endoscopy in 3 horses demonstrated both its potential usefulness and limitations. An equine specific prototype intended to address some of these limitations is currently under development by one of the authors (KW).
Acknowledgments
We acknowledge the following undergraduate students for their help with the horses during the trial period: Louisa Belgrave, Samantha Steinke, Colby Klein, and Joscelyn McKenzie. Dr. Wahid’s laboratory is funded by the Natural Sciences and Engineering Research Council of Canada. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Redondo-Cerezo E. Wireless capsule endoscopy: Perspectives beyond gastrointestinal bleeding. World J Gastroenterol. 2014;20:15 664–15 673. doi: 10.3748/wjg.v20.i42.15664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song HJ, Shim K-N. Current status and future perspectives of capsule endoscopy. Intest Res. 2016;14:21–29. doi: 10.5217/ir.2016.14.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Enns RA, Hookey L, Armstrong D, et al. Clinical practice guidelines for the use of video capsule endoscopy. Gastroenterology. 2017;152:497–514. doi: 10.1053/j.gastro.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 4.Lee ACY, Epe C, Simpson KW, Bowman DD. Utility of capsule endoscopy for evaluating anthelmintic efficacy in fully conscious dogs. Int J Parasitol. 2011;41:1377–1383. doi: 10.1016/j.ijpara.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Lee ACY, Hostetler JA, Bowman DD. Assessing the speed of kill of hookworms, Ancylostoma caninum, by Advantage Multi® for dogs using endoscopic methods. Vet Parasitol. 2014;204:402–406. doi: 10.1016/j.vetpar.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 6.Davignon DL, Lee ACY, Johnston AN, Bowman DD, Simpson KW. Evaluation of capsule endoscopy to detect mucosal lesions associated with gastrointestinal bleeding in dogs. J Small Anim Pract. 2016;57:148–158. doi: 10.1111/jsap.12442. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki N, Yamada H. Preliminary study of capsule endoscopy in the small intestine of horses. Aust Vet J. 2010;88:342–345. doi: 10.1111/j.1751-0813.2010.00612.x. [DOI] [PubMed] [Google Scholar]
- 8.Khan TH, Shrestha R, Wahid KA, Babyn P. Design of a smart-device and FPGA based wireless capsule endoscopic system. Sens Actuators A Phys. 2015;221:77–87. [Google Scholar]
- 9.Sainju S, Bui F, Wahid KA. Automated bleeding detection in capsule endoscopy videos using statistical features and region growing. J Med Syst. 2014;38:25. doi: 10.1007/s10916-014-0025-1. [DOI] [PubMed] [Google Scholar]