Figure 1.
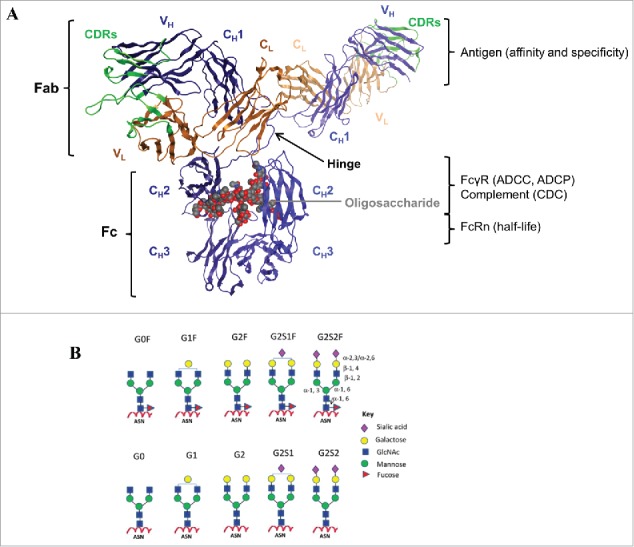
IgG structure and function. (A) The crystal structure of a human IgG1 molecule is used to illustrate domain assignments (PDB ID: 1HZH). An individual IgG is composed of 2 identical heavy chains (blue) and 2 identical light chains (orange), linked together by inter-chain disulfide bonds. Each heavy chain consists of a variable (VH) domain and 3 constant (CH1, CH2, and CH3) domains, while the light chain is composed of a variable (VL) and a constant (CL) domain. The light chain pairs with the VH and CH1 domains to form the Fab, which interacts to form the antigen-binding region, also known as the complementarity-determining region (CDR, green). The CH2 and CH3 domains dimerize to form the Fc, which is connected to the upper Fab region via a flexible hinge containing several disulfide bridges that covalently link the CH1 and CH2 chains together. The interactions between the Fc and the Fc-gamma receptors (FcγR) expressed by effector cells or the complement component 1q (C1q), are vital to the clearance of target antigens through antibody-dependent cell-mediated cytotoxicity (ADCC) and phagocytosis (ADCP), or complement-dependent cytotoxicity (CDC), respectively. The highly conserved N-linked glycosylation site (gray) located in the CH2 domains is responsible for the overall structural integrity of the IgG molecule to mediate effector functions. In addition, the Fc can bind the neonatal Fc receptor (FcRn) in a strictly pH-dependent manner, an interaction that contributes to the long serum half-lives of human IgGs. (B) Schematic diagrams of N-linked glycoforms commonly found in human IgGs or industrial mAbs. The N-linked glycoforms attached to asparagine (Asn) 297 are categorized into 2 groups: fucosylated (top panels) and non-fucosylated (bottom panels). A core heptasaccharide structure (G0) is composed of 2 N-acetylglucosamine (GlcNAc), 3 mannose, and 2 GlcNAc residues that are β-1,2 linked to the mannose from the α-1,6 and α-1,3 arms, forming 2 antennae. In addition to fucose, galactose, bisecting GlcNAc, and sialic acid may be added to the core. Figure reproduced from18 with permission from Elsevier.
