Figure 3.
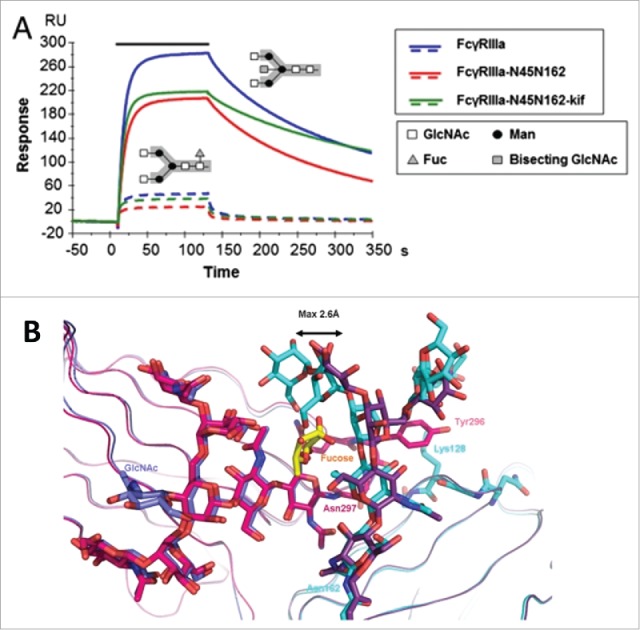
Fucosylation-induced allostery on FcγR binding. (A) Comparison of the binding interactions between FcγRIIIA and human IgG1 glycovariants. Overlay of SPR sensorgrams for binding of 125 nM FcγRIIIA glycovariants to fucosylated (dotted line) and afucosylated (continuous line) IgG1s. The association phase is indicated by a solid bar above the curves. The afucosylated IgG significantly enhanced binding to all FcγRIIIA glycovariants with up to 100-fold increase in affinity as compared with the fucosylated version. The N-linked glycosylation is shown in the insert containing the core pentasaccharide (gray box) and the additional carbohydrate residues (legend box). (B) Overlay of the crystal interaction interface between glycosylated FcγRIIIA and Fc glycovariants. Chain A of the afucosylated (blue) bound to FcγRIIIA (cyan) and of the fucosylated (magenta) Fc bound to FcγRIIIA (dark violet) with core fucose (yellow). The oligosaccharide at Asn 162 is displaced by a maximum distance of 2.6 Å in comparison to its position in the structure with a fucosylated Fc. Figure reproduced from an open access article from.79
