Figure 4.
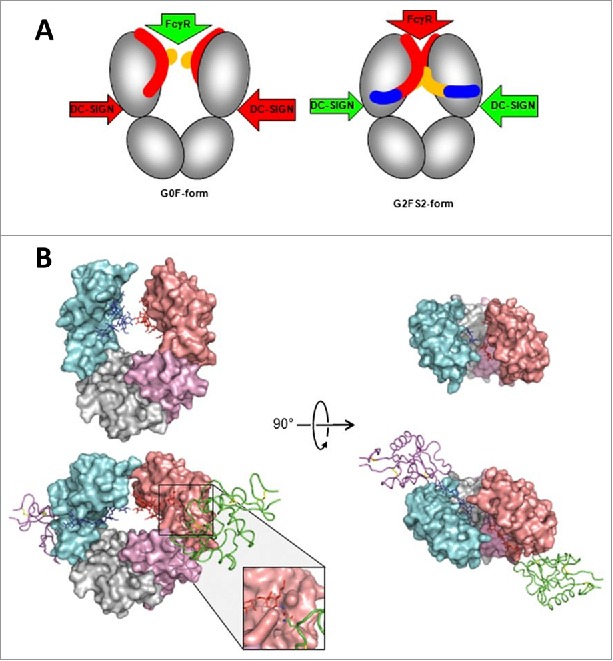
A proposed allosteric model for Fc effector function regulation through sialylation. (A) Schematic of the blockade of FcγR binding as a result of sialylation-induced conformational changes within the Fc. The G0F-Fc maintains an “open” conformation (left) that allows FcγR binding, whereas the fully sialylated G2FS2 (blue) interacts with the CH2 domain to induce a “closed” conformation that prevents FcγR binding while revealing the binding site for DC-SIGN. DC-SIGN is an alternative cellular receptor responsible for anti-inflammatory responses (please refer to the article for details). (B) The “open” crystal front (upper left) and top (upper right) view of the G0F-Fc and the “closed” crystal front (lower left) and top (lower right) view of the G2FS2-Fc with DC-SIGN bound. Figure reproduced from82 with permission from PNAS.
