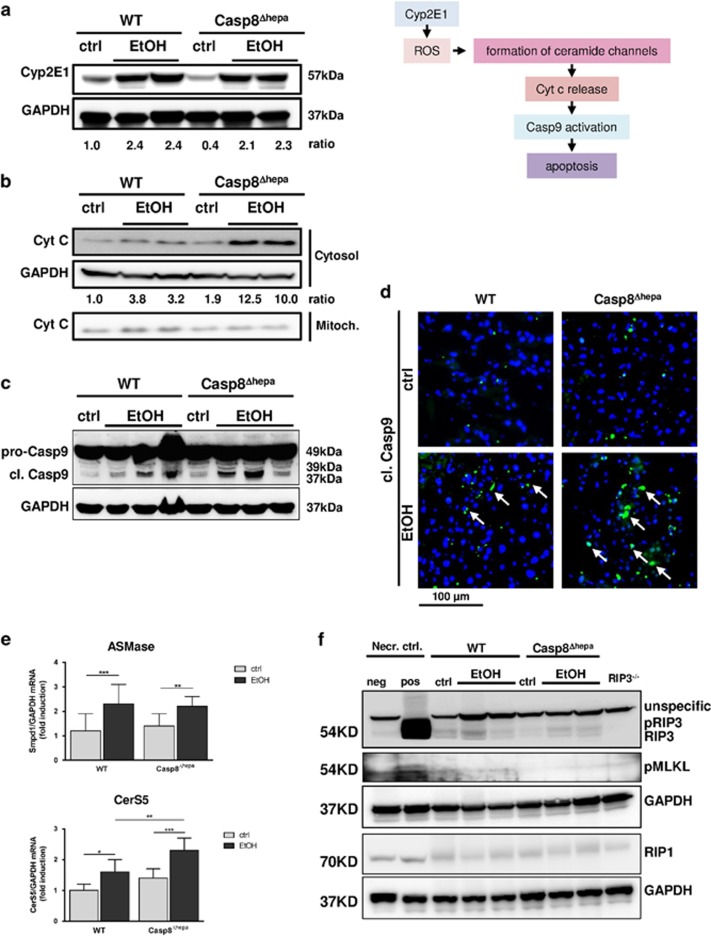
Figure 5.
Loss of Caspase-8 enforces intrinsic apoptosis signaling after EtOH feeding. (a) Left: Immunoblot analysis of CYP2E1 in the liver of WT and Casp8Δhepa mice kept on Lieber-DeCarli diet for 8 weeks. Right: Rationale for the subsequent measurements. We hypothesized that ethanol-driven apoptosis in Casp8Δhepa mice could involve de-regulated ceramide biosynthesis and enhanced cytochrome c release from mitochondria thereby inducing increased Caspase-9-mediated intrinsic apoptosis. Accordingly, the indicated intermediate steps were systematically investigated by immunoblot analysis, immunofluorescence stainings and qPCR. (b) Western blot analysis of cytochrome c levels in cytosolic and mitochondrial liver fractions. (c) Immunoblot analysis of cleaved Caspase-9 in the liver of WT and Casp8Δhepa mice. Quantification of Western blots was performed by densitometry. (d) Assessment of Caspase-9 activation in situ through fluorescence microscopy. Liver cryosections were stained with an antibody specific for activated (i.e. cleaved; cl.) Caspase-9. Cytoplasmic, activated Caspase-9 is stained in green and highlighted with arrows; total nuclei are counter stained with DAPI (blue). (e) qPCR analysis of ASMase and CerS5 mRNA expression. *P<0.05**P<0.01; ***P<0.01. (f) Analysis of hepatic necroptosis by immunoblot analysis of RIP3, RIP1 and pMLKL expression. As a positive control for necroptosis (Necr. ctrl., pos), murine embryonic fibroblasts (MEF) were co-stimulated with the pan-caspase inhibitor Z-VAD-FMK (20 μM) and TNF (100 ng/ml) resulting in substantial induction of RIP3 and necroptotic cell death. As a negative control for necroptosis, proteins from untreated MEF (Necr. ctrl., neg) and a whole liver extract from RIP3 knockout mice (RIP3−/−) were used