Abstract
Osteoarthritis (OA) is an inflammatory joint disease characterized by degeneration of articular cartilage within synovial joints. An estimated 27 million Americans suffer from OA, and the population is expected to reach 67 million in the United States by 2030. Thus, it is urgent to find an effective treatment for OA. Traditional OA treatments have no disease-modifying effect, while regenerative OA therapies such as autologous chondrocyte implantation show some promise. Nonetheless, current regenerative therapies do not overcome synovial inflammation that suppresses the differentiation of mesenchymal stem cells (MSCs) to chondrocytes and the expression of type II collagen, the major constituent of functional cartilage. We discovered a synergistic combination that overcame synovial inflammation to form type II collagen-producing chondrocytes. The combination consists of peroxisome proliferator–activated receptor (PPAR) δ agonist, human bone marrow (hBM)-derived MSCs, and hyaluronic acid (HA) gel. Interestingly, those individual components showed their own strong enhancing effects on chondrogenesis. GW0742, a PPAR-δ agonist, greatly enhanced MSC chondrogenesis and the expression of type II collagen and glycosaminoglycan (GAG) in hBM-MSC-derived chondrocytes. GW0742 also increased the expression of transforming growth factor β that enhances chondrogenesis and suppresses cartilage fibrillation, ossification, and inflammation. HA gel also increased MSC chondrogenesis and GAG production. However, neither GW0742 nor HA gel could enhance the formation of type II collagen-producing chondrocytes from hBM-MSCs within human OA synovial fluid. Our data demonstrated that the combination of hBM-MSCs, PPAR-δ agonist, and HA gel significantly enhanced the formation of type II collagen-producing chondrocytes within OA synovial fluid from 3 different donors. In other words, the novel combination of PPAR-δ agonist, hBM-MSCs, and HA gel can overcome synovial inflammation to form type II collagen cartilage within human OA synovial fluid. This novel articularly injectable formula could improve OA treatment in the future clinical application.
Keywords: PPAR-δ agonist, osteoarthritis, mesenchymal stem cells, synovial inflammation, hyaluronic acid, type II collagen
Introduction
Osteoarthritis (OA) currently affects 12% of the US population (21 million Americans aged 25 and older), but there is no disease-modifying agent for OA treatment. OA is manifested by gradual loss of cartilage extracellular matrix (ECM) by inflammatory degradation, resulting in joint dysfunction. There have been various approaches to treat OA, for example, autologous chondrocyte implantation (ACI) and use of nonsteroidal anti-inflammatory drugs (NSAIDs), but none of those yield satisfactory outcomes. Moreover, those treatments are followed by unwanted results. For example, ACI leads to the morbidity of the donor site and the formation of nonfunctional fibrocartilage.1–3 Conversely, chronic treatment with NSAIDs causes serious side effects such as gastrointestinal bleeding and cardiovascular diseases.4,5 Thus, it is of high urgency to find a better treatment for OA.
Recently, mesenchymal stem cells (MSCs) have arisen as a better treatment option for OA6 due to their ability to differentiate into chondrocytes and release growth factors that regulate the immune response through paracrine effects.7,8 Intra-articular MSC injection has shown some promise in OA treatment.9 Nonetheless, it appears that MSCs alone are not sufficient to satisfy the desired properties of an OA treatment agent.10 An agent with such properties for an OA therapeutic is not yet available.
Current MSC-based OA therapy is limited by the absence of a strong chondrogenic inducer and a stable scaffold. The other major problem that decreases the efficacy of MSC-based OA therapies is synovial inflammation,11–13 which blocks MSC chondrogenesis14–17 and type II collagen expression.18,19 Thus, if MSC therapy is strengthened with a strong chondrogenic inducer, a stable scaffold, and the ability to overcome inflammation, it is expected to improve the outcome of OA.
OA is an inflammatory joint disease characterized by degeneration of articular cartilage within synovial joints, which is accompanied by synovitis, subchondral bone remodeling, and osteophyte formation.20,21 An estimated 27 million Americans, especially aged people,22 are diagnosed with OA,23 and the affected population is expected to increase to 67 million in the United States by 2030.24 Unfortunately, traditional OA treatments including physical therapy, NSAIDs, hyaluronic acid (HA) gel injections, and arthroscopic surgery have no disease-modifying effect.25,26 Thus, finding more effective treatment is of utmost urgency.
Healthy cartilage in the joint is maintained by nonproliferating chondrocytes that generate ECM consisting of type II collagen and glycosaminoglycan (GAG)27 within hypoxic synovial environment that facilitates chondrogenesis.28–30 Transforming growth factor β (TGF-β),31 bone morphogenetic proteins (BMPs) such as BMP-232 and BMP-4,33 and fibroblast growth factor 2 (FGF2)34,35 drive the differentiation of MSCs to type II collagen chondrocytes. Sometimes, chondrocytes dedifferentiate into type I collagen-generating chondrocytes in monolayer culture and make fibrous cartilage36 which is often observed after ACI and microfracture.37,38 Chondrocytes also undergo terminal differentiation into hypertrophic chondrocytes that calcify cartilage by increasing alkaline phosphatase (ALP) and runt-related transcriptional factor 2 (Runx2) and undergo apoptosis, resulting in cartilage ossification and vascularization.39,40 TGF-β and parathyroid hormone–related peptide block the hypertrophic differentiation.41–43
Mechanical damage and/or age-related wear and tear are thought to trigger systematic inflammatory responses in all tissues surrounding the joint including articular cartilage, synovial membrane, subchondral bone, and ligaments.44,45 Cartilage debris generated by cartilage degradation increases synovial inflammation by activating synovial macrophages.46,47 Synovial inflammation then accelerates cartilage degradation by increasing matrix metalloproteases (MMPs) and aggrecanases47–49 and reduces cartilage generation by blocking the formation of chondrocytes14–17 and the production of type II collagen and GAG.18,19 Thus, to modify OA, synovial inflammation should be controlled.50
The strong immunomodulatory properties of MSCs have been used for anti-inflammatory treatment51–57 and regenerative therapy.58–61 OA is one of the major clinical targets for MSC therapy.62–68 We believe that MSC-based OA therapies have not shown clear success yet69 because of the following problems: (1) the absence of a strong chondrogenesis inducer, (2) the absence of a stable scaffold that increases the efficiency of chondrocyte differentiation, and (3) the inability of MSCs to overcome inflammation.15–18
To solve the first problem, several chondrogenic factors have been tested in clinical trials by co-injecting agents such as BMP-7 or sprifermin with MSCs into human OA joints70,71; however, the outcome was not satisfactory. We tested the peroxisome proliferator–activated receptor (PPAR) δ agonist, GW0742, regarding its chondrogenic effect on human bone marrow (hBM) MSCs during our study since this PPAR-δ agonist is reported to induce the expression of TGF-β,72 which is known to facilitate the formation of functional cartilage consisting of type II collagen in early chondrogenesis.31 In later chondrogenesis, TGF-β is required for the maintenance of functional cartilage by suppressing the terminal differentiation of chondrocytes.43,73 GW0742 is a highly selective PPAR-δ agonist; the half maximal effective concentration (EC50) of GW0742 for PPAR-δ is ∼1 nM with 1,000-fold selectivity over PPAR-α in human.74,75 Thus, if a PPAR-δ agonist is able to increase TGF-β as expected, it should be a good chondrogenic factor for MSCs.
We examined HA gel8,76 regarding its efficacy as a scaffold for MSC chondrogenesis. Various scaffolding polymers have been tested in combination with MSCs or chondrocytes for articular injection,63,77–83 and some showed positive outcomes.79,81–87 Compared to other scaffolds, HA gel88 has several advantages: (1) HA gel provides cushion to joints for pain relief and functional improvement89; (2) HA gel facilitates the infiltration, migration, and repopulation of MSCs and chondrocytes90–92; and (3) HA gel protects cartilage ECM from oxidative degradation93 and MMPs.94 Therefore, HA gel should provide those advantages for MSC chondrogenesis in addition to its chondroprotective effect.
Current MSC-based therapy has no solution for the third problem caused by synovial inflammation.14–19 Given that both MSCs7,8 and PPAR-δ agonists95–98 have anti-inflammatory effects, the combination of those should have additive anti-inflammatory effects on OA joints.
In this study, we demonstrate how the combination of a PPAR-δ agonist, hBM-MSCs, and HA gel overcomes OA synovial inflammation to generate type II collagen-producing chondrocytes.
Materials and Methods
Isolation of hBM-MSCs
Frozen hBM-derived mononuclear cells were purchased from AllCells (Alameda, CA, USA) and used for isolation of hBM-derived MSCs.99 After thawing the cells, mononuclear cells were resuspended in α-minimum essential medium (MEM; Life Technologies, Thermo Fisher Scientific, Grand Island, NY, USA) plus 20% fetal bovine serum (FBS; Sigma-Aldrich, St Louis, MO, USA) and 1% antibiotic–antimycotic solution (Invitrogen, Carlsbad, CA, USA), plated at a density of 1 × 106 cells/75 cm2 flask, and incubated in the same media. Nucleated cells were plated at a density of 1 to 5 × 106 cells per 75 cm2 flask and cultured at 37 °C in a 5% CO2 incubator. The medium over hBM-MSCs was exchanged with fresh medium after 48 h and every 3 to 4 d for 14 d.
Chondrogenesis
Chondrogenic differentiation was performed by high-density micromass culturing. hBM-MSCs (passages 2–3) were plated in a 75 cm2 flask at a density of 1.5 × 106 cells/cm2 and cultured in α-MEM with 20% FBS for 1 d at 37 °C. On the next day, hBM-MSCs were harvested, and 80,000 cells were seeded on 70 μL HA gel/well (Euflexxa, 1% sodium hyaluronate; Ferring Pharmaceuticals Inc., Parsippany, NJ, USA) in a 96-well plate for 2 h at 37 °C and incubated in 50 µL chondrogenesis medium (STEMPRO chondrogenesis differentiation kit; Life Technologies, Thermo Fisher Scientific). Medium was changed every 2 d. Clinical HA gels used for 3-dimensional (3-D) culture included Euflexxa (Ferring Pharmaceuticals Inc.), Synvisc (Genzyme Biosurgery, Ridgefield, NJ, USA), Orthovisc (Anika Therapeutics, Inc., Bedford, MA, USA), or Supartz (Seikagaku Co., Tokyo, Japan). After chondrocytes formed spheroids, the medium over the spheroids was replaced with fresh chondrogenesis medium every 2 d and incubated for 14 d for chondrogenesis. In order to identify the effective dose of GW0742 (Cayman Chemical, Ann Arbor, MI, USA) for chondrogenesis, 0.0001, 0.001, 0.01, 0.1, or 1 μM GW0742 was added to hBM-MSCs in chondrogenic media. In addition, to examine whether the mixture of hBM-MSCs, GW0742, and/or HA gel forms type II collagen-generating chondrocytes in the synovial fluids of OA patients, the mixture of 0.3 × 106 hBM-MSCs, 1 μM GW0742, and/or 70 μL Euflexxa was incubated in chondrogenic medium with 50% human OA synovial fluid for 14 d. For a control, 50% phosphate-buffered saline (PBS) was added instead of human OA synovial fluid. To eliminate donor-to-donor variability, we used synovial fluid from 3 different OA patients and used BM-MSCs from 3 donors (AllCells) to measure the GAG content. The use of human synovial fluid for our study was approved by the institutional review board of the University of Toledo. Discarded synovial fluids were obtained from two 65-year-old patients and one 57-year-old patient after obtaining their informed consent.
Quantification of GAG Production
The extent of GAG production was examined by labeling GAG with Alcian blue (Sigma-Aldrich). The chondrocyte pellet in each well was washed once with PBS (Sigma-Aldrich), fixed with 4% formaldehyde in PBS for 30 min, and stained with 1% Alcian blue in 0.1 N HCl (Sigma-Aldrich) for 30 min. Cell pellets were washed 3 times with 0.1 N HCl and then with distilled water for pH neutralization. The images of GAG staining of cell pellets were taken using a light microscope. The number, size, and blue color intensity of cell pellets were quantified using Metamorph software (Molecular Devices, Inc., Downingtown, PA, USA). In addition, the content of total sulfated GAG in chondrocyte spheroids was quantified by modified dimethyl-methylene (DMM) blue method. Briefly, chondrocyte spheroids were digested in papain buffer (55 mM sodium citrate [Sigma-Aldrich], 5 mM ethylenediaminetetraacetic acid [EDTA; Sigma-Aldrich], 150 mM NaCl [Sigma-Aldrich], 0.56 U/mL papain [Sigma-Aldrich], pH 6.8) at 60 °C overnight. Forty microliter aliquot of the digestion was mixed with 200 μL of DMM blue dye solution (Sigma-Aldrich). Then, the absorbance at 595 nm of each well was measured using a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA, USA) equipped with the software, SoftMax Pro 5.2 (Molecular Probes, Eugene, OR, USA). The amounts of GAG were extrapolated from a standard curve using shark chondroitin sulfate (Sigma-Aldrich). Quantification was normalized by DNA content that was measured by spectrophotometry.
Quantification of Protein Expression
Chondrocyte pellets were stored at −80 °C until assayed. Frozen samples were pulverized under liquid nitrogen and placed in a homogenization buffer (10 mM phosphate buffer, 250 mM sucrose, 1 mM EDTA, 0.1 mM phenylmethanesulfonyl fluoride or phenylmethylsulfonyl fluoride, and 0.1% [v/v] Tergitol, pH 7.5). Homogenates were centrifuged at 27,000g for 10 min at 4 °C to harvest supernatant for immunoblotting with antibodies. Immunoblotting was performed using primary monoclonal antibodies against PPAR-δ (1:2,000 dilution; Abcam, Cambridge, MA, USA), type II collagen (1:1,000 dilution; EMD Millipore, Billerica, MA, USA), TGF-β (1:1,000 μg/mL; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and β-actin (1:10,000 dilution; Abcam) and secondary antibodies tagged horseradish peroxidase (HRP) (rabbit for PPAR-δ and TGF-β, 1:20,000 dilution, mouse for type II collagen and β-actin, 1:5,000 dilution). Cell pellets were processed for NuPAGE gel (Invitrogen). Proteins separated in the gel were transferred to nitrocellulose membrane (GE Healthcare Life Sciences, Pittsburgh, PA, USA) using TE77XP semidry blotter with 10 V for 3 h (Hoefer, Inc., Holliston, MA, USA). Protein band signals on blots were detected on Amersham Hyperfilm enhanced chemiluminescence (GE Healthcare Life Sciences) using SuperSignal West Pico Chemiluminescent Substrate kit (Thermo Fisher Scientific, Rockford, IL, USA).
Staining Chondrocytes
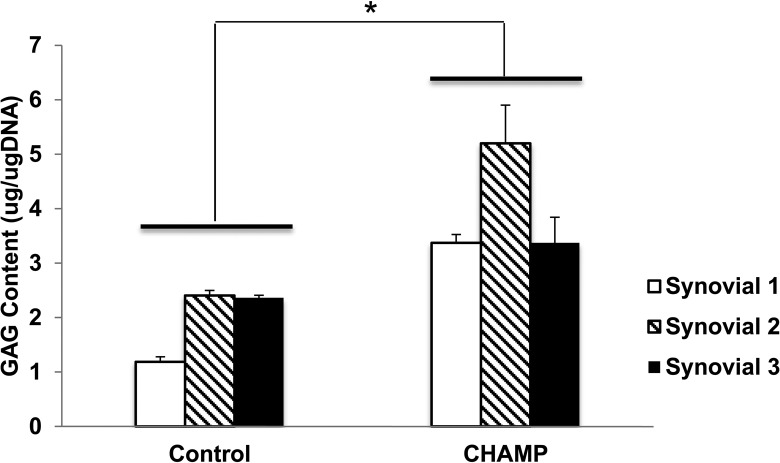
On day 14, spheroids in 96 wells were transferred to 1.5 microtubes and stained as follows. Briefly, 1 μg/mL DiO (Dye Aye; Invitrogen) cell-labeling solutions and anticollagen II antibody (1:5,000 dilution, EMD Millipore) were added directly to the tube with normal culture media to label. Cell suspensions were incubated for 5 min at 37 °C. After incubation, the cells were spun down, rinsed, and resuspended in fresh medium. Secondary antigoat fluorescein isothiocyanate (1:1,000 dilution, mouse for type II collagen; Abcam) was added. Images were taken with Cytation 5 (BioTek Instruments, Inc., Winooski, VT, USA) and confocal images were obtained with CellVoyager CV1000 (Olympus, Center Valley, PA, USA).
Statistical Analysis
All cell culture experiments were replicated multiple times with different batches of cell cultures. Microscopic analysis was performed blindly by students. Statistical significance between 2 groups was calculated using unpaired Student t tests and 2-way analysis of variance (ANOVA). Tukey’s post hoc test was performed. A value of P < 0.05 was considered statistically significant. All statistical analysis was performed with GraphPad Prism 5 (GraphPad, San Diego, CA, USA).
Results
Culturing in HA Gel Enhances Chondrocyte Spheroid Formation and GAG Production
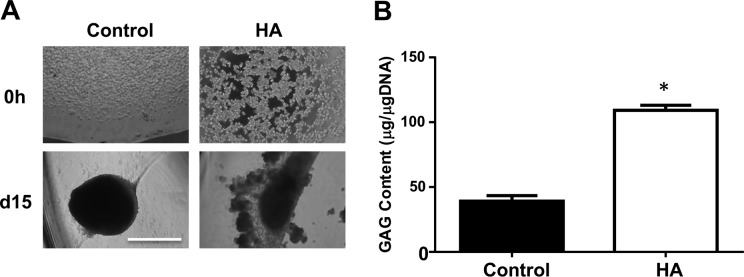
MSCs are aggregated and condensed for chondrogenesis,100 forming “chondrocyte spheroids” in vitro (Fig. 1A). Given that culturing within HA gel provides more contacts between ECM and chondrogenic receptors on MSCs,101,102 it should enhance MSC chondrogenesis to a higher extent than those on plastic walls. To examine this possibility, hBM-MSCs were seeded either on CC2-treated plastic walls (2-D culture) or in 70 μL of the clinical HA gel (Euflexxa, 3-D culture) in the wells of a 96-well plate and incubated in STEMPRO chondrogenesis differentiation medium for 15 d. Chondrocytes in HA gel formed larger spheroids than those on plastic walls (Fig. 1). We then quantified the amount of produced GAG by DMM blue assay. Chondrocytes and their surrounding ECM were harvested and processed to quantify GAG. The amount of GAG was extrapolated from a standard curve using shark chondroitin sulfate and normalized by DNA content. There was a significant elevation in GAG production in the chondrocytes in HA gel compared to those on plastic walls (Fig. 1B). These results suggest that 3-D culturing in HA gel is better for MSC chondrogenesis and GAG production than 2-D culturing on plastic walls.
Figure 1.
Culturing in hyaluronic acid (HA) gel plus peroxisome proliferator–activated receptor (PPAR) δ agonist enhances chondrogenesis and the formation of type II collagen and transforming growth factor (TGF) β. (A) Human bone marrow–derived mesenchymal stem cells (hBM-MSCs) were cultured either on a plastic wall or in 70 μL of HA gel during chondrogenesis. Cell pictures (100×) were taken after 0- and 15-d chondrogenesis. (B) The amount of glycosaminoglycan (GAG) produced by chondrocytes was quantified by modified dimethyl-methylene (DMM) method and normalized by DNA content. *P < 0.01 versus control. The image is the representative of 3 independent experiments. Data presented is the result of 3 independent experiments. Scale bar: 80 μm.
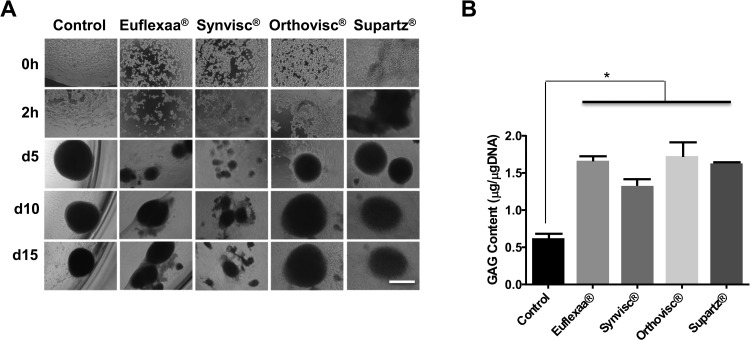
In addition, we examined whether different types of clinically used HA gels enhance chondrogenesis to different extents (Fig. 2). After 5 d of chondrogenesis, chondrocytes in Orthovisc and Supartz formed the spheroids of medium sizes (diameters [Φ] = 20–30 μm), while those in Euflexxa and Synvisc formed small spheroids ([Φ] < 20 μm). We also performed DMM blue assays to measure GAG production in different HA gels. There was no significant difference in the amounts of GAG production among different HA gels (Fig. 2B). Nonetheless, 3-D culturing of MSCs in any type of HA gel produced more GAG than those on plastic walls. Together, Euflexxa appears to be slightly better at inducing spheroid formation than the other HA gels, but not in GAG production.
Figure 2.
(A) Human bone marrow–derived mesenchymal stem cells (hBM-MSCs) were cultured in 70 μL of clinical hyaluronic acid (HA) gel during chondrogenesis in 96-well plates. Cell pictures (100×) were taken at 0 and 2 h and on 5, 10, and 15 d on the gels. (B) hBM-MSCs were cultured in 70 μL of different HA gels during chondrogenesis in 96-well plates. On day 15, the content of glycosaminoglycan (GAG) of chondrocytes was measured with modified dimethyl-methylene (DMM) blue assay at 525 nm. The amount of GAG was extrapolated from a standard curve using shark chondroitin sulfate. n = 3. *P < 0.05 versus control. Image is the representation of 3 independent experiments. Data presented are the result of 3 independent experiments. Scale bar: 80 μm.
Treatment with PPAR-δ Agonist Increases the Production of Type II Collagen, TGF-β, and GAG
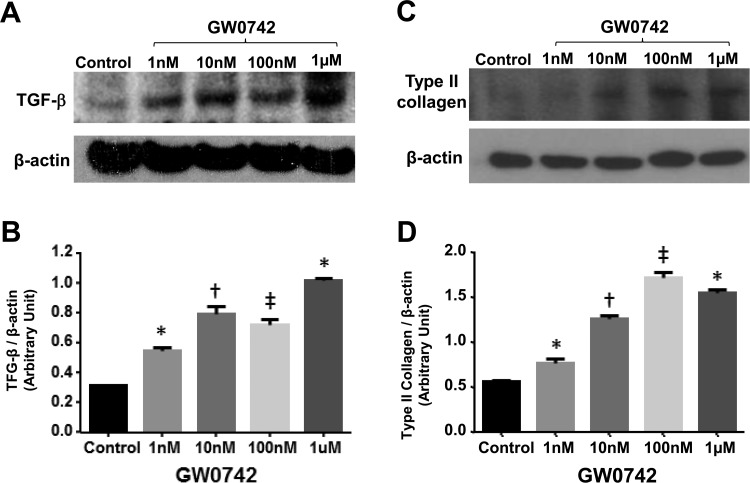
Given that the PPAR-δ agonist induces the expression of TGF-β,72 a good chonodrogenesis enhancer,31 we examined whether PPAR-δ agonist can function as chondrogenic agent for MSCs. First, we examined the extent to which incubation of hBM-MSCs with GW0742, a PPAR-δ agonist, increases TGF-β production. We treated hBM-MSCs in HA gel (Euflexxa) every second day with 0, 1, 10, 100 nM, or 1 μM of GW0742 in chondrogenic media for 15 d. Then, chondrocytes were processed for immunoblotting using the antibodies to type II collagen and TGF-β (β-actin: loading control). GW0742 increased the protein level of TGF-β in a dose-dependent manner with a peak at 0.1 to 1 μM (Fig. 3A, B), suggesting that the PPAR-δ agonist, GW0742, is a strong inducer of TGF-β. Then, we examined whether GW0742 enhances the expression of type II collagen as it did for TGF-β. GW0742 indeed increased the protein level of type II collagen in a dose-dependent manner with a peak at 0.1 to 1 μM (Fig. 3C, D). These results suggest that the PPAR-δ agonist is a strong inducer of TGF-β and type II collagen.
Figure 3.
Human bone marrow–derived mesenchymal stem cells (hBM-MSCs) were cultured in 70 μL of Euflexxa during chondrogenesis in 96-well plates. On day 7, the expression of type II collagen and transforming growth factor (TGF) β in chondrocytes was detected by immunoblotting using antibodies to type II collagen (A) and TGF-β (B; anti-β-actin antibody as control). The relative levels of type II collagen (C) and TGF-β (D) to β-actin were quantified by densitometry (ImageJ [from the National Institutes of Health in Bethesda, MD, USA https://imagej.nih.gov/ij/]) and presented in bar graphs (n = 3). *P < 0.05 versus control. †P < 0.005 versus control. ‡P < 0.001 versus control. Image is the representative of 3 independent experiments. Data presented are the result of 3 independent experiments.
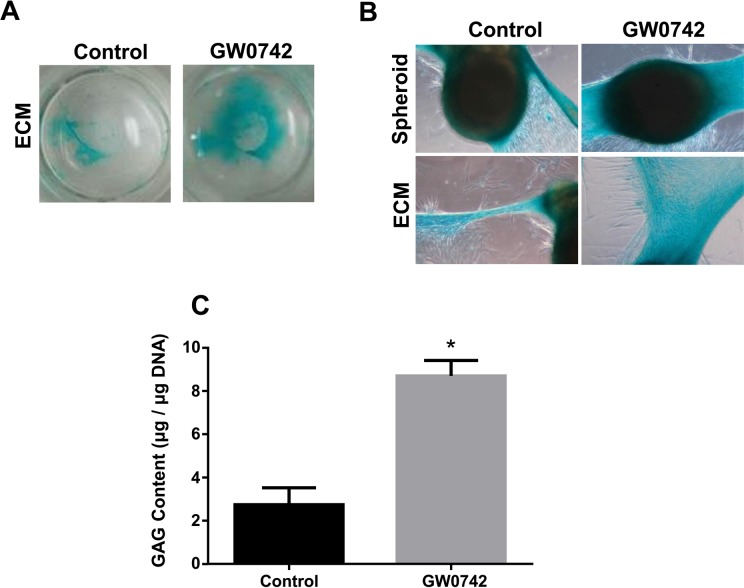
Then, we examined the effect of GW0742 on GAG production by Alcian blue staining (Fig. 4). Since Alcian blue also stains HA gel, we did not use HA gel for this experiment. hBM-MSCs on plastic walls in chondrogenic medium were treated with 0.1 μM GW0742 for 14 d. Chondrocytes formed without GW0742 generated only a small amount of GAG, while those with GW0742 produced a significant amount of GAG (Fig. 4B, C ). We quantified the extent of GAG production around spheroid by measuring the blue-stained area occupied by ECM and the intensity of GAG staining using Metamorph software. The area occupied by GAG produced by GW0742-treated chondrocytes was ∼2-fold bigger than that by untreated chondrocytes (Fig. 4C).
Figure 4.
GW0742 enhances glycosaminoglycan (GAG) production. Human bone marrow–derived mesenchymal stem cells (hBM-MSCs) in chondrogenic medium were treated with vehicle (dimethyl sulfoxide [DMSO]) or GW0742 (0.1 μM) every 2 d in a 24-well plate. On day 14, GAGs were stained with 1% Alcian blue. The images of Alcian blue staining of the whole well or individual spheroids were taken by a light microscope. (C) The amount of GAG produced by chondrocytes was quantified by modified dimethyl-methylene (DMM) method and normalized by DNA content. *P < 0.005 versus control. Image is the representative of 3 independent experiments. Data presented are the result of 3 independent experiments.
Thus, it is clear that the PPAR-δ agonist, GW0742, exerts a strong chondrogenic effect by enhancing the formation of functional chondrocytes that generate a significant amount of type II collagen, TGF-β, and GAG.
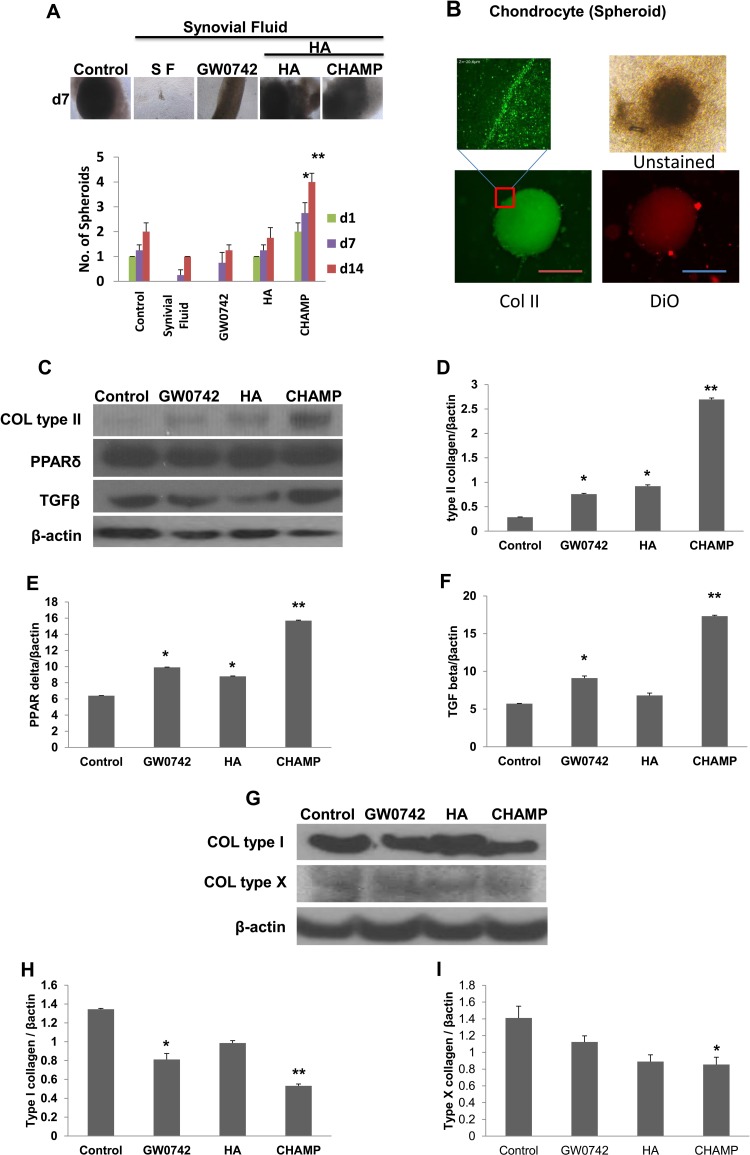
The Combination of GW0742, hBM-MSCs, and HA Gel Forms Chondrocytes That Express High Levels of Type II Collagen and TGF-β within Human OA Synovial Fluid
Based on the strong chondrogenic effect of the PPAR-δ agonist and HA gel and the innate immunosuppressive effect of MSCs and PPAR-δ agonist, we speculated that the combination of hBM-MSCs with either GW0742 or HA gel might overcome synovial inflammation to form functional type II collagen-producing chondrocytes. Thus, we examined whether those combinations could generate type II collagen-producing chondrocytes within human OA synovial fluid. We incubated 0.3 × 106 hBM-MSCs on 70 μL Euflexxa for 2 h. When cells aggregated, we added 1 μM GW0742 to chondrogenic medium + 50% human OA synovial fluid and incubated the combinations for 14 d. Fifty percent chondrogenic media was added as control and 50% PBS as vehicles. Then, we examined the extent of chondrogenesis by counting the number of chondrocyte spheroids and quantifying the expression levels of type II collagen and TGF-β. hBM-MSCs alone on plastic walls formed ∼1 chondrocyte spheroid after 14-d chondrogenesis in the absence of human OA synovial fluid (Fig. 5A). Spheroids were stained with anti-type II collagen antibody to confirm cells were chondrocytes, and the expression of type II collagen was confirmed using confocal and fluorescent microplates (Fig. 5B).
Figure 5.
Human bone marrow–derived mesenchymal stem cells (hBM-MSCs) were incubated in chondrogenic medium without (control) and with GW0742, hyaluronic acid (HA), or Chondrogenic Hyaluronic Acid–Mesenchymal Stem Cells–PPAR-δ agonist (CHAMP) in 50% of human osteoarthritis (OA) synovial fluid for 14 d. The numbers of chondrocyte spheroid (>40 mm) were counted for bar graphs (A). The spheroids were stained with fluorescent agent (DiO) and collagen II were stained with fluorescein isothiocyanate (FITC) to confirm chondrocytes. The magnified image was taken using confocal microscope (B). On day 14, chondrocytes were processed for immunoblotting using antibodies to type II collagen, peroxisome proliferator–activated receptor (PPAR) δ, transforming growth factor (TGF) β (C–F), type I collagen, type X collagen, and β-actin (G–I). Protein band densities were measured to obtain bar graphs (C–I). Mean ± standard error, n = 3. *P < 0.05 versus control. **P < 0.01 versus control. Image is the representative of 3 independent experiments. Data presented are the result of 3 independent experiments. Scale bar: 80 μm.
However, the group treated with 50% chondrogenic media and synovial fluid only could not form a noticeable spheroid within OA synovial fluid, suggesting that hBM-MSCs alone on plastic walls are not sufficient enough to form chondrocyte spheroids. Next, we added either GW0742 or HA gel to hBM-MSCs grown on plastic walls and incubated them in human OA synovial fluid. Addition of GW0742 alone could not cause the formation of a large spheroid on plastic walls within OA synovial fluid (Fig. 5A). Conversely, incubation of MSCs within HA gel and OA synovial fluid could form ∼1 large spheroid similar to MSCs alone on plastic wall in the absence of the fluid, while it did not show the enhancement of chondrogenesis by Euflexxa in the absence of the fluid (Fig. 5A). Therefore, neither the PPAR-δ agonist nor HA gel alone is sufficient to enhance MSC chondrogenesis within OA synovial fluid.
Then, we mixed GW0742, hBM-MSCs, and HA gel together prior to incubation in human OA synovial fluid. We named the mixture Chondrogenic Hyaluronic Acid-Mesenchymal Stem Cells-PPAR-δ agonist (CHAMP). Strikingly, the combination of hBM-MSCs, GW0742, and HA gel formed multiple large chondrocyte spheroids (Fig. 5A), suggesting that CHAMP can overcome OA synovial inflammation to form multiple chondrocyte spheroids.
In addition to spheroid counting, we quantified the protein expression levels of type II collagen, PPAR-δ, TGF-β, and β-actin (β-actin was loading control for normalization) in chondrocytes formed in different conditions (Fig. 5C–F). MSCs alone on plastic walls did not express noticeable amounts of type II collagen, PPAR-δ, or TGF-β after 14-d chondrogenesis. Within synovial fluid, hBM-MSCs on plastic walls expressed PPAR-δ and TGF-β but not type II collagen. Addition of either GW0742 or HA gel to hBM-MSCs slightly increased the expression levels of PPAR-δ, TGF-β, and type II collagen. In contrast, the complete combination of hBM-MSCs, GW0742, and HA gel (CHAMP) significantly increased the protein expression of PPAR-δ, TGF-β, and type II collagen within human OA synovial fluid (Fig. 5C–F). Addition of either GW0742 or CHAMP to hBM-MSCs slightly decreased the expression levels of type I collagen (Fig. 5G). Type X collagen within human OA synovial fluid was decreased in CHAMP (Fig. 5I).
GAG Content in CHAMP
GAG content was elevated in CHAMP within 3 different human OA synovial fluids (Fig. 6). Taken together, our results clearly demonstrate that the combination of hBM-MSCs, GW0742, and HA gel (CHAMP) is capable of forming chondrocytes that express type II collagen and TGF-β within human OA synovial fluid.
Figure 6.
The amount of glycosaminoglycan (GAG) produced by chondrocytes was quantified by modified dimethyl-methylene (DMM) method and normalized by DNA content with 3 different synovial fluids from patients. *P < 0.01 versus control.
Discussion
Current MSC-based OA therapy is limited by the absence of strong chondrogenic inducers and stable scaffolds for chondrogenesis. The other major problem that decreases the efficacy of MSC-based OA therapies is synovial inflammation11–13 that blocks MSC chondrogenesis14–17 and type II collagen expression.18,19 Thus, if MSC therapy is strengthened with strong chondrogenic inducers, stable scaffolds, and an ability to overcome inflammation, it is expected to improve the outcome of MSC-based OA therapy.
We found that the highly selective PPAR-δ agonist, GW0742, has strong chondrogenesis-enhancing properties. It significantly increased the production of type II collagen and GAG, two main components of functional cartilage, in hBM-MSC-derived chondrocytes. It also increased the expression of TGF-β, the key regulator that enhances chondrogenesis and suppresses the formation of fibrous and ossified cartilage.43,73 In addition, we found that the 3-D environment inside of the HA gel scaffold enhanced MSC chondrogenesis. The 3-D environment appears to provide more hypoxic conditions for chondrogenesis.103 Moreover, the 3-D environment appears to facilitate the condensation of chondrocytes,104 thus forming chondrocyte spheroids that simulate the cellular condensation required for embryonic mesenchymal chondrogenesis by providing the physical and biochemical cues conducive to cartilage formation.100 Thus, both the PPAR-δ agonist and HA gel have great advantageous properties to enhance chondrogenesis.
Spheroids are well formed when BM-MSCs are grown on HA and produce GAG, but it may be controversial as to whether these spheroids are chondrocytes. Therefore, we isolated these spheroids and stained them with anti-type II collagen antibody, confirming that type II collagen, a marker of articular cartilage,105 was highly expressed (Fig. 5B).
In spite of those individual advantageous properties, the combination of hBM-MSCs with either GW0742 or HA gel could not form type II collagen-producing chondrocytes efficiently within human OA synovial fluid. The anti-inflammatory properties of MSCs106–108 and GW074295–98,109,110 were not enough to overcome synovial inflammation. On the other hand, when GW0742, hBM-MSCs, and HA gel were mixed together, the mixture greatly enhanced the chondrogenesis of hBM-MSCs, thus forming type II collagen-producing chondrocytes within human OA synovial fluid. It appears that the combination in CHAMP generates a strong synergism to overcome the inflammatory environment, thus forming type II collagen-producing chondrocytes.
Type II collagen expression is associated with early condensation when MSCs differentiate into chondrocytes that express BMPs.111 Type X collagen is expressed in the hypertrophic zone and is expressed in the calcification of the interstitial matrix during endochondral ossification (which means terminal differentiation).112 Thus, the reduction of type I and X collagens by GW0742, HA, or CHAMP found in this study was significant. While GW0742, HA, or CHAMP inhibited terminal differentiation or degeneration of chondrocytes (Fig. 5G–I), CHAMP increased type II collagen or GAG content at the same time (Figs. 5C and 6). These findings imply that CHAMP could be a potential therapy for OA.
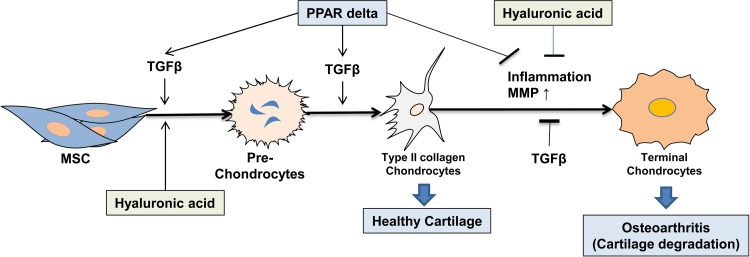
Based on previous findings and findings from the current study regarding CHAMP components, we built a model to ascertain how those components work together, thus forming type II collagen-producing chondrocytes inside inflamed joints (Fig. 7). PPAR-δ agonist increases TGF-β that, in turn, facilitates MSC chondrogenesis with the assistance of HA gel, resulting in formation of type II collagen-producing chondrocytes. Later on, the PPAR-δ agonist, TGF-β, and HA gel suppress inflammation and degradation of cartilage. In our opinion, all of these actions together were expected to exert an OA-modifying effect.
Figure 7.
Working model: transforming growth factor (TGF) β, peroxisome proliferator–activated receptor (PPAR) δ and hyaluronic acid (HA) greatly enhance mesenchymal stem cell (MSC) chondrogenesis to type II collagen-producing chondrocytes that constitute healthy cartilage. MSCs, PPAR-δ, TGF-β, and HA suppress inflammation and matrix metalloproteases (MMPs) in inflamed osteoarthritis (OA) joints, thus blocking terminal chondrocyte differentiation, which should reduce cartilage degradation and calcification that occur inside OA joint.
Transplantation of MSCs + HA gel as a treatment for OA has been demonstrated in preclinical and clinical studies.8,76,90,92 Thus, our articularly injectable formula with the addition of a PPAR-δ agonist to MSCs + HA gel should be easily transplantable into the joint. Moreover, since HA gel has properties that make it a good drug carrier for controlled release,113–117 GW0742 was expected to be slowly released from HA gel during its priming of MSCs for chondrogenesis after it is injected along with MSCs + HA gel into the joint. That is, one can easily reproduce the efficiency of CHAMP transplantation in animal models that we demonstrated in vitro. Therefore, this study is of extreme importance as it showed a signaling molecule, that is, PPAR-δ agonist, that enhanced functional cartilage formation in an inflammatory environment of OA if it is injected with MSCs + HA gel into OA-affected synivial fluid.
Conclusions
First, we found a new property of PPAR-δ agonist to enhance chondrogenesis. Second, we discovered a novel combination of PPAR-δ agonist, HA gel, and hBM-MSCs (CHAMP) that could form type II collagen-producing chondrocytes within human OA synovial fluid. The combination is actually designed for articular injection into the synovial cavity such that it is easily applicable in clinics if use is approved for clinical trials in the future. Fortunately, all 3 components of CHAMP are in clinical trials or already available in clinics so the regulatory process for their entry into clinics is expected to be short. Our future studies will focus on the efficacy of CHAMP in treating OA in animal models, which may be consistent with the clinical outcome in the future.
Footnotes
Author Contributions: D.H.K. contributed to conception and design, data analysis and interpretation, provision of study material, and manuscript writing. J.J.P. contributed to data analysis and interpretation and manuscript writing. V.M. assisted with data collection and analysis. B.E.H. contributed to conception and design, financial support, data analysis and interpretation, provision of study material, and manuscript writing. E.-C.K. contributed data analysis and interpretation, provision of study material, and manuscript writing.
Ethical Approval: The use of human synovial fluid for our study was approved by the institutional review board of the University of Toledo.
Statement of Human and Animal Rights: This study was approved by the institutional review board of the University of Toledo and informed patient consent was obtained.
Statement of Informed Consent: Discarded synovial fluids were obtained from patients after obtaining their informed consent.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported by NWO Stem Cure, LLC. This work also was supported by the Engineering Research Center of Excellence Program of Korea Ministry of Science, ICT & Future Planning (MSIP)/National Research Foundation of Korea (NRF; Grant NRF-2016R1D1A1B03935879).
References
- 1. Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89(10):2105–2112. [DOI] [PubMed] [Google Scholar]
- 2. McNickle AG, L’Heureux DR, Yanke AB, Cole BJ. Outcomes of autologous chondrocyte implantation in a diverse patient population. Am J Sports Med. 2009;37(7):1344–1350. [DOI] [PubMed] [Google Scholar]
- 3. Pelttari K, Steck E, Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury. 2008;39(suppl 1):S58–S65. [DOI] [PubMed] [Google Scholar]
- 4. Farkouh ME, Kirshner H, Harrington RA, Ruland S, Verheugt FW, Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, et al. ; TARGET Study Group. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet. 2004;364(9435):675–684. [DOI] [PubMed] [Google Scholar]
- 5. Lazzaroni M, Bianchi Porro G. Gastrointestinal side-effects of traditional non-steroidal anti-inflammatory drugs and new formulations. Aliment Pharmacol Ther. 2004;20(Suppl 2):48–58. [DOI] [PubMed] [Google Scholar]
- 6. Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7(6):259–264. [DOI] [PubMed] [Google Scholar]
- 7. Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzon IM, Nepomnaschy I, Costa H, Canones C, Raiden S, Vermeulen M, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5(2):e9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48(12):3464–3474. [DOI] [PubMed] [Google Scholar]
- 9. Jeong SY, Kim DH, Ha J, Jin HJ, Kwon SJ, Chang JW, Choi SJ, Oh W, Yang YS, Kim G, et al. Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells. 2013;31(10):2136–2148. [DOI] [PubMed] [Google Scholar]
- 10. Wyles CC, Houdek MT, Atta Behfar A, Sierra RJ. Mesenchymal stem cell therapy for osteoarthritis: current perspectives. Stem Cells Cloning. 2015;8:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beekhuizen M, Bastiaansen-Jenniskens YM, Koevoet W, Saris DB, Dhert WJ, Creemers LB, van Osch GJ. Osteoarthritic synovial tissue inhibition of proteoglycan production in human osteoarthritic knee cartilage: establishment and characterization of a long-term cartilage-synovium coculture. Arthritis Rheum. 2011;63(7):1918–1927. [DOI] [PubMed] [Google Scholar]
- 12. Bondeson J, Blom AB, Wainwright S, Hughes C, Caterson B, van den Berg WB. The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 2010;62(3):647–657. [DOI] [PubMed] [Google Scholar]
- 13. Orlowsky EW, Kraus VB. The role of innate immunity in osteoarthritis: when our first line of defense goes on the offensive. J Rheumatol. 2015;42(3):363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Felka T, Schafer R, Schewe B, Benz K, Aicher WK. Hypoxia reduces the inhibitory effect of IL-1beta on chondrogenic differentiation of FCS-free expanded MSC. Osteoarthritis Cartilage. 2009;17(10):1368–1376. [DOI] [PubMed] [Google Scholar]
- 15. Heldens GT, Blaney Davidson EN, Vitters EL, Schreurs BW, Piek E, van den Berg WB, van der Kraan PM. Catabolic factors and osteoarthritis-conditioned medium inhibit chondrogenesis of human mesenchymal stem cells. Tissue Eng Part A. 2011;18(1-2):45–54. [DOI] [PubMed] [Google Scholar]
- 16. Ousema PH, Moutos FT, Estes BT, Caplan AI, Lennon DP, Guilak F, Weinberg JB. The inhibition by interleukin 1 of MSC chondrogenesis and the development of biomechanical properties in biomimetic 3D woven PCL scaffolds. Biomaterials. 2012;33(35):8967–8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wehling N, Palmer GD, Pilapil C, Liu F, Wells JW, Muller PE, Evans CH, Porter RM. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009;60(3):801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goldring MB, Fukuo K, Birkhead JR, Dudek E, Sandell LJ. Transcriptional suppression by interleukin-1 and interferon-gamma of type II collagen gene expression in human chondrocytes. J Cell Biochem. 1994;54(1):85–99. [DOI] [PubMed] [Google Scholar]
- 19. Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986;322(6079):547–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoff P, Buttgereit F, Burmester GR, Jakstadt M, Gaber T, Andreas K, Matziolis G, Perka C, Rohner E. Osteoarthritis synovial fluid activates pro-inflammatory cytokines in primary human chondrocytes. Int Orthop. 2012;37(1):145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2):77–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthritis Cartilage. 2009;17(8):971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, et al. ; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bitton R. The economic burden of osteoarthritis. Am J Manag Care. 2009;15(suppl 8): S230–S235. [PubMed] [Google Scholar]
- 25. Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571–576. [DOI] [PubMed] [Google Scholar]
- 26. Jevsevar DS, Brown GA, Jones DL, Matzkin EG, Manner PA, Mooar P, Schousboe JT, Stovitz S, Sanders JO, Bozic KJ, et al. ; American Academy of Orthopaedic Surgeons. The American Academy of Orthopaedic Surgeons evidence-based guideline on: treatment of osteoarthritis of the knee, 2nd edition J Bone Joint Surg Am. 2013;95(20):1885–1886. [DOI] [PubMed] [Google Scholar]
- 27. Freyria AM, Mallein-Gerin F. Chondrocytes or adult stem cells for cartilage repair: the indisputable role of growth factors. Injury. 2012;43(3):259–265. [DOI] [PubMed] [Google Scholar]
- 28. Kalpakci KN, Brown WE, Hu JC, Athanasiou KA. Cartilage tissue engineering using dermis isolated adult stem cells: the use of hypoxia during expansion versus chondrogenic differentiation. PLoS One. 2014;9(5):e98570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kanichai M, Ferguson D, Prendergast PJ, Campbell VA. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. J Cell Physiol. 2008;216(3):708–715. [DOI] [PubMed] [Google Scholar]
- 30. Koay EJ, Athanasiou KA. Hypoxic chondrogenic differentiation of human embryonic stem cells enhances cartilage protein synthesis and biomechanical functionality. Osteoarthritis Cartilage. 2008;16(12):1450–1456. [DOI] [PubMed] [Google Scholar]
- 31. Danisovic L, Lesny P, Havlas V, Teyssler P, Syrova Z, Kopani M, Fujerikova G, Trc T, Sykova E, Jendelova P. Chondrogenic differentiation of human bone marrow and adipose tissue-derived mesenchymal stem cells. J Appl Biomed. 2007;5(3):139–150. [Google Scholar]
- 32. Sekiya I, Larson BL, Vuoristo JT, Reger RL, Prockop DJ. Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell Tissue Res. 2005;320(2):269–276. [DOI] [PubMed] [Google Scholar]
- 33. Miljkovic ND, Cooper GM, Marra KG. Chondrogenesis, bone morphogenetic protein-4 and mesenchymal stem cells. Osteoarthritis Cartilage. 2008;16(10):1121–1130. [DOI] [PubMed] [Google Scholar]
- 34. Ling L, Nurcombe V, Cool SM. Wnt signaling controls the fate of mesenchymal stem cells. Gene. 2009;433(1–2):1–7. [DOI] [PubMed] [Google Scholar]
- 35. Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI, Welter JF. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. J Cell Physiol. 2005;203(2):398–409. [DOI] [PubMed] [Google Scholar]
- 36. Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30(1):215–224. [DOI] [PubMed] [Google Scholar]
- 37. Falah M, Nierenberg G, Soudry M, Hayden M, Volpin G. Treatment of articular cartilage lesions of the knee. Int Orthop. 2010;34(5):621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang L, Hu J, Athanasiou KA. The role of tissue engineering in articular cartilage repair and regeneration. Crit Rev Biomed Eng. 2009;37(1-2):1–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hennig T, Lorenz H, Thiel A, Goetzke K, Dickhut A, Geiger F, Richter W. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211(3):682–691. [DOI] [PubMed] [Google Scholar]
- 40. Pelttari K, Winter A, Steck E, Goetzke K, Hennig T, Ochs BG, Aigner T, Richter W. Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice. Arthritis Rheum. 2006;54(10):3254–3266. [DOI] [PubMed] [Google Scholar]
- 41. Kim YJ, Kim HJ, Im GI. PTHrP promotes chondrogenesis and suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs. Biochem Biophys Res Commun. 2008;373(1):104–108. [DOI] [PubMed] [Google Scholar]
- 42. Mwale F, Yao G, Ouellet JA, Petit A, Antoniou J. Effect of parathyroid hormone on type X and type II collagen expression in mesenchymal stem cells from osteoarthritic patients. Tissue Eng Part A. 2010;16(11):3449–3455. [DOI] [PubMed] [Google Scholar]
- 43. Weiss S, Hennig T, Bock R, Steck E, Richter W. Impact of growth factors and PTHrP on early and late chondrogenic differentiation of human mesenchymal stem cells. J Cell Physiol. 2010;223(1):84–93. [DOI] [PubMed] [Google Scholar]
- 44. Goldring MB. Articular cartilage degradation in osteoarthritis. HSS J. 2013;8(1):7–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pearle AD, Warren RF, Rodeo SA. Basic science of articular cartilage and osteoarthritis. Clin Sports Med. 2005;24(1):1–12. [DOI] [PubMed] [Google Scholar]
- 46. Heinegard D, Saxne T. The role of the cartilage matrix in osteoarthritis. Nat Rev Rheumatol. 2010;7(1):50–56. [DOI] [PubMed] [Google Scholar]
- 47. Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6(11):625–635. [DOI] [PubMed] [Google Scholar]
- 48. Lefebvre V, Peeters-Joris C, Vaes G. Modulation by interleukin 1 and tumor necrosis factor alpha of production of collagenase, tissue inhibitor of metalloproteinases and collagen types in differentiated and dedifferentiated articular chondrocytes. Biochim Biophys Acta. 1990;1052(3):366–378. [DOI] [PubMed] [Google Scholar]
- 49. Mengshol JA, Vincenti MP, Coon CI, Barchowsky A, Brinckerhoff CE. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43(4):801–811. [DOI] [PubMed] [Google Scholar]
- 50. Attur M, Samuels J, Krasnokutsky S, Abramson SB. Targeting the synovial tissue for treating osteoarthritis (OA): where is the evidence? Best Pract Res Clin Rheumatol. 2010;24(1):71–79. [DOI] [PubMed] [Google Scholar]
- 51. Farini A, Sitzia C, Erratico S, Meregalli M, Torrente Y. Clinical applications of mesenchymal stem cells in chronic diseases. Stem Cells Int. 2014;2014:306573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gharibi T, Ahmadi M, Seyfizadeh N, Jadidi-Niaragh F, Yousefi M. Immunomodulatory characteristics of mesenchymal stem cells and their role in the treatment of multiple sclerosis. Cell Immunol. 2015;293(2):113–121. [DOI] [PubMed] [Google Scholar]
- 53. Griffin MD, Ritter T, Mahon BP. Immunological aspects of allogeneic mesenchymal stem cell therapies. Hum Gene Ther. 2010;21(12):1641–1655. [DOI] [PubMed] [Google Scholar]
- 54. Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014;6(5):552–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Maumus M, Guerit D, Toupet K, Jorgensen C, Noel D. Mesenchymal stem cell-based therapies in regenerative medicine: applications in rheumatology. Stem Cell Res Ther. 2011;2(2):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Paek HJ, Kim C, Williams SK. Adipose stem cell-based regenerative medicine for reversal of diabetic hyperglycemia. World J Diabetes. 2014;5(3):235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van der Marel S, Majowicz A, van Deventer S, Petry H, Hommes DW, Ferreira V. Gene and cell therapy based treatment strategies for inflammatory bowel diseases. World J Gastrointest Pathophysiol. 2011;2(6):114–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Asatrian G, Pham D, Hardy WR, James AW, Peault B. Stem cell technology for bone regeneration: current status and potential applications. Stem Cells Cloning. 2015;2015(8):39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Baghaban Eslaminejad M, Malakooty Poor E. Mesenchymal stem cells as a potent cell source for articular cartilage regeneration. World J Stem Cells. 2014;6(3):344–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dave S. Mesenchymal stem cells derived in vitro transdifferentiated insulin-producing cells: a new approach to treat type 1 diabetes. Adv Biomed Res. 2014;3:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wakao S, Matsuse D, Dezawa M. Mesenchymal stem cells as a source of Schwann cells: their anticipated use in peripheral nerve regeneration. Cells Tissues Organs. 2014;200(1):31–41. [DOI] [PubMed] [Google Scholar]
- 62. Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36(4):568–584. [DOI] [PubMed] [Google Scholar]
- 63. Bornes TD, Adesida AB, Jomha NM. Mesenchymal stem cells in the treatment of traumatic articular cartilage defects: a comprehensive review. Arthritis Res Ther. 2015;16(5):432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Davatchi F, Abdollahi BS, Mohyeddin M, Shahram F, Nikbin B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 2011;14(2):211–215. [DOI] [PubMed] [Google Scholar]
- 65. Koelling S, Miosge N. Stem cell therapy for cartilage regeneration in osteoarthritis. Expert Opin Biol Ther. 2009;9(11):1399–1405. [DOI] [PubMed] [Google Scholar]
- 66. Mobasheri A, Csaki C, Clutterbuck AL, Rahmanzadeh M, Shakibaei M. Mesenchymal stem cells in connective tissue engineering and regenerative medicine: applications in cartilage repair and osteoarthritis therapy. Histol Histopathol. 2009;24(3):347–366. [DOI] [PubMed] [Google Scholar]
- 67. Noth U, Steinert AF, Tuan RS. Technology insight: adult mesenchymal stem cells for osteoarthritis therapy. Nat Clin Pract Rheumatol. 2008;4(7):371–380. [DOI] [PubMed] [Google Scholar]
- 68. Vinatier C, Bouffi C, Merceron C, Gordeladze J, Brondello JM, Jorgensen C, Weiss P, Guicheux J, Noel D. Cartilage tissue engineering: towards a biomaterial-assisted mesenchymal stem cell therapy. Curr Stem Cell Res Ther. 2009;4(4):318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Steinert AF, Rackwitz L, Gilbert F, Noth U, Tuan RS. Concise review: the clinical application of mesenchymal stem cells for musculoskeletal regeneration: current status and perspectives. Stem Cells Transl Med. 2012;1(3):237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hunter DJ, Pike MC, Jonas BL, Kissin E, Krop J, McAlindon T. Phase 1 safety and tolerability study of BMP-7 in symptomatic knee osteoarthritis. BMC Musculoskelet Disord. 2010;11(232):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lohmander LS, Hellot S, Dreher D, Krantz EF, Kruger DS, Guermazi A, Eckstein F. Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol. 2014;66(7):1820–1831. [DOI] [PubMed] [Google Scholar]
- 72. Kim HJ, Ham SA, Kim SU, Hwang JY, Kim JH, Chang KC, Yabe-Nishimura C, Seo HG. Transforming growth factor-beta1 is a molecular target for the peroxisome proliferator-activated receptor delta. Circ Res. 2008;102(2):193–200. [DOI] [PubMed] [Google Scholar]
- 73. Zhang X, Ziran N, Goater JJ, Schwarz EM, Puzas JE, Rosier RN, Zuscik M, Drissi H, O’Keefe RJ. Primary murine limb bud mesenchymal cells in long-term culture complete chondrocyte differentiation: TGF-beta delays hypertrophy and PGE2 inhibits terminal differentiation. Bone. 2004;34(5):809–817. [DOI] [PubMed] [Google Scholar]
- 74. Graham TL, Mookherjee C, Suckling KE, Palmer CN, Patel L. The PPARdelta agonist GW0742X reduces atherosclerosis in LDLR(-/-) mice. Atherosclerosis. 2005;181(1):29–37. [DOI] [PubMed] [Google Scholar]
- 75. Sznaidman ML, Haffner CD, Maloney PR, Fivush A, Chao E, Goreham D, Sierra ML, LeGrumelec C, Xu HE, Montana VG, et al. Novel selective small molecule agonists for peroxisome proliferator-activated receptor delta (PPARdelta)--synthesis and biological activity. Bioorg Med Chem Lett. 2003;13(9):1517–1521. [DOI] [PubMed] [Google Scholar]
- 76. Sato M, Uchida K, Nakajima H, Miyazaki T, Guerrero AR, Watanabe S, Roberts S, Baba H. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res Ther. 2012;14(1):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Buda R, Vannini F, Cavallo M, Grigolo B, Cenacchi A, Giannini S. Osteochondral lesions of the knee: a new one-step repair technique with bone-marrow-derived cells. J Bone Joint Surg Am. 2010;92(Suppl 2):2–11. [DOI] [PubMed] [Google Scholar]
- 78. Enea D, Cecconi S, Calcagno S, Busilacchi A, Manzotti S, Kaps C, Gigante A. Single-stage cartilage repair in the knee with microfracture covered with a resorbable polymer-based matrix and autologous bone marrow concentrate. Knee. 2013;20(6):562–569. [DOI] [PubMed] [Google Scholar]
- 79. Giannini S, Buda R, Cavallo M, Ruffilli A, Cenacchi A, Cavallo C, Vannini F. Cartilage repair evolution in post-traumatic osteochondral lesions of the talus: from open field autologous chondrocyte to bone-marrow-derived cells transplantation. Injury. 2010;41(11):1196–1203. [DOI] [PubMed] [Google Scholar]
- 80. Gigante A, Cecconi S, Calcagno S, Busilacchi A, Enea D. Arthroscopic knee cartilage repair with covered microfracture and bone marrow concentrate. Arthrosc Tech. 2013;1(2):e175–e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Haleem AM, Singergy AA, Sabry D, Atta HM, Rashed LA, Chu CR, El Shewy MT, Azzam A, Abdel Aziz MT. The clinical use of human culture-expanded autologous bone marrow mesenchymal stem cells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects: a pilot study and preliminary results. Cartilage. 2010;1(4):253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kuroda R, Ishida K, Matsumoto T, Akisue T, Fujioka H, Mizuno K, Ohgushi H, Wakitani S, Kurosaka M. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthritis Cartilage. 2007;15(2):226–231. [DOI] [PubMed] [Google Scholar]
- 83. Wakitani S, Nawata M, Tensho K, Okabe T, Machida H, Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007;1(1):74–79. [DOI] [PubMed] [Google Scholar]
- 84. Giannini S, Buda R, Battaglia M, Cavallo M, Ruffilli A, Ramponi L, Pagliazzi G, Vannini F. One-step repair in talar osteochondral lesions: 4-year clinical results and t2-mapping capability in outcome prediction. Am J Sports Med. 2012;41(3):511–518. [DOI] [PubMed] [Google Scholar]
- 85. Giannini S, Buda R, Vannini F, Cavallo M, Grigolo B. One-step bone marrow-derived cell transplantation in talar osteochondral lesions. Clin Orthop Relat Res. 2009;467(12):3307–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kasemkijwattana C, Hongeng S, Kesprayura S, Rungsinaporn V, Chaipinyo K, Chansiri K. Autologous bone marrow mesenchymal stem cells implantation for cartilage defects: two cases report. J Med Assoc Thai. 2011;94(3):395–400. [PubMed] [Google Scholar]
- 87. Nejadnik H, Hui JH, Feng Choong EP, Tai BC, Lee EH. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med. 2010;38(6):1110–1116. [DOI] [PubMed] [Google Scholar]
- 88. Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103(4):655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lieberman JR, Engstrom SM, Solovyova O, Au C, Grady JJ. Is intra-articular hyaluronic acid effective in treating osteoarthritis of the hip joint? J Arthroplasty. 2014;30(3):507–511. [DOI] [PubMed] [Google Scholar]
- 90. Aulin C, Bergman K, Jensen-Waern M, Hedenqvist P, Hilborn J, Engstrand T. In situ cross-linkable hyaluronan hydrogel enhances chondrogenesis. J Tissue Eng Regen Med. 2011;5(8): e188–e196. [DOI] [PubMed] [Google Scholar]
- 91. Levett PA, Melchels FP, Schrobback K, Hutmacher DW, Malda J, Klein TJ. Chondrocyte redifferentiation and construct mechanical property development in single-component photocrosslinkable hydrogels. J Biomed Mater Res A. 2014;102(8):2544–2553. [DOI] [PubMed] [Google Scholar]
- 92. Responte DJ, Natoli RM, Athanasiou KA. Identification of potential biophysical and molecular signalling mechanisms underlying hyaluronic acid enhancement of cartilage formation. J R Soc Interface. 2012;9(77):3564–3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yasui T, Akatsuka M, Tobetto K, Hayaishi M, Ando T. The effect of hyaluronan on interleukin-1 alpha-induced prostaglandin E2 production in human osteoarthritic synovial cells. Agents Actions. 1992;37(1-2):155–156. [DOI] [PubMed] [Google Scholar]
- 94. Campo GM, Avenoso A, Campo S, Ferlazzo AM, Altavilla D, Calatroni A. Efficacy of treatment with glycosaminoglycans on experimental collagen-induced arthritis in rats. Arthritis Res Ther. 2003;5(3): R122–R131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Di Paola R, Briguglio F, Paterniti I, Mazzon E, Oteri G, Militi D, Cordasco G, Cuzzocrea S. Emerging role of PPAR-beta/delta in inflammatory process associated to experimental periodontitis. Mediators Inflamm. 2011;2011:787159. doi:10.1155/2011/787159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kapoor A, Shintani Y, Collino M, Osuchowski MF, Busch D, Patel NS, Sepodes B, Castiglia S, Fantozzi R, Bishop-Bailey D, et al. Protective role of peroxisome proliferator-activated receptor-beta/delta in septic shock. Am J Respir Crit Care Med. 2010;182(12):1506–1515. [DOI] [PubMed] [Google Scholar]
- 97. Matsushita Y, Ogawa D, Wada J, Yamamoto N, Shikata K, Sato C, Tachibana H, Toyota N, Makino H. Activation of peroxisome proliferator-activated receptor delta inhibits streptozotocin-induced diabetic nephropathy through anti-inflammatory mechanisms in mice. Diabetes. 2011;60(3):960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Takata Y, Liu J, Yin F, Collins AR, Lyon CJ, Lee CH, Atkins AR, Downes M, Barish GD, Evans RM, et al. PPARdelta-mediated antiinflammatory mechanisms inhibit angiotensin II-accelerated atherosclerosis. Proc Natl Acad Sci U S A. 2008;105(11):4277–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kim DH, Liu J, Bhat S, Benedict G, Lecka-Czernik B, Peterson SJ, Ebraheim NA, Heck BE. Peroxisome proliferator-activated receptor delta agonist attenuates nicotine suppression effect on human mesenchymal stem cell-derived osteogenesis and involves increased expression of heme oxygenase-1. J Bone Miner Metab. 2013;31(1):44–52. [DOI] [PubMed] [Google Scholar]
- 100. Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265–272. [DOI] [PubMed] [Google Scholar]
- 101. Julovi SM, Ito H, Nishitani K, Jackson CJ, Nakamura T. Hyaluronan inhibits matrix metalloproteinase-13 in human arthritic chondrocytes via CD44 and P38. J Orthop Res. 2011;29(2):258–264. [DOI] [PubMed] [Google Scholar]
- 102. Knudson CB, Knudson W. Hyaluronan and CD44: modulators of chondrocyte metabolism. Clin Orthop Relat Res. 2004;(Suppl 427):S152–S162. [PubMed] [Google Scholar]
- 103. Sheehy EJ, Buckley CT, Kelly DJ. Oxygen tension regulates the osteogenic, chondrogenic and endochondral phenotype of bone marrow derived mesenchymal stem cells. Biochem Biophys Res Commun. 2011;417(1):305–310. [DOI] [PubMed] [Google Scholar]
- 104. Aigner J, Tegeler J, Hutzler P, Campoccia D, Pavesio A, Hammer C, Kastenbauer E, Naumann A. Cartilage tissue engineering with novel nonwoven structured biomaterial based on hyaluronic acid benzyl ester. J Biomed Mater Res. 1998;42(2):172–181. [DOI] [PubMed] [Google Scholar]
- 105. Goldring MB. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther Adv Musculoskelet Dis. 2012;4(4):269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Djouad F, Fritz V, Apparailly F, Louis-Plence P, Bony C, Sany J, Jorgensen C, Noel D. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen-induced arthritis. Arthritis Rheum. 2005;52(5):1595–1603. [DOI] [PubMed] [Google Scholar]
- 107. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, et al. ; Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–1586. [DOI] [PubMed] [Google Scholar]
- 108. Zhou K, Zhang H, Jin O, Feng X, Yao G, Hou Y, Sun L. Transplantation of human bone marrow mesenchymal stem cell ameliorates the autoimmune pathogenesis in MRL/lpr mice. Cell Mol Immunol. 2008;5(6):417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Collino M, Benetti E, Miglio G, Castiglia S, Rosa AC, Aragno M, Thiemermann C, Fantozzi R. Peroxisome proliferator-activated receptor beta/delta agonism protects the kidney against ischemia/reperfusion injury in diabetic rats. Free Radic Biol Med. 2010;50(2):345–353. [DOI] [PubMed] [Google Scholar]
- 110. Galuppo M, Di Paola R, Mazzon E, Esposito E, Paterniti I, Kapoor A, Thiemermann C, Cuzzocrea S. GW0742, a high affinity PPAR-beta/delta agonist reduces lung inflammation induced by bleomycin instillation in mice. Int J Immunopathol Pharmacol. 2011;23(4):1033–1046. [DOI] [PubMed] [Google Scholar]
- 111. Onyekwelu I, Goldring MB, Hidaka C. Chondrogenesis, joint formation, and articular cartilage regeneration. J Cell Biochem. 2009;107(3):383–392. [DOI] [PubMed] [Google Scholar]
- 112. Harrington EK, Lunsford LE, Svoboda KK. Chondrocyte terminal differentiation, apoptosis, and type X collagen expression are downregulated by parathyroid hormone. Anat Rec A Discov Mol Cell Evol Biol. 2004;281(2):1286–1295. [DOI] [PubMed] [Google Scholar]
- 113. Khatun Z, Nurunnabi M, Nafiujjaman M, Reeck GR, Khan HA, Cho KJ, Lee YK. A hyaluronic acid nanogel for photo-chemo theranostics of lung cancer with simultaneous light-responsive controlled release of doxorubicin. Nanoscale. 2015;7(24):10680–10689. [DOI] [PubMed] [Google Scholar]
- 114. Li NN, Fu CP, Zhang LM. Using casein and oxidized hyaluronic acid to form biocompatible composite hydrogels for controlled drug release. Mater Sci Eng C Mater Biol Appl. 2014;36:287–293. [DOI] [PubMed] [Google Scholar]
- 115. Mayol L, Biondi M, Russo L, Malle BM, Schwach-Abdellaoui K, Borzacchiello A. Amphiphilic hyaluronic acid derivatives toward the design of micelles for the sustained delivery of hydrophobic drugs. Carbohydr Polym. 2014;102:110–116. [DOI] [PubMed] [Google Scholar]
- 116. Widjaja LK, Bora M, Chan PN, Lipik V, Wong TT, Venkatraman SS. Hyaluronic acid-based nanocomposite hydrogels for ocular drug delivery applications. J Biomed Mater Res A. 2014;102(9):3056–3065. [DOI] [PubMed] [Google Scholar]
- 117. Yu Y, Lau LC, Lo AC, Chau Y. Injectable chemically crosslinked hydrogel for the controlled release of bevacizumab in vitreous: a 6-month in vivo study. Transl Vis Sci Technol. 2015;4(2):5. [DOI] [PMC free article] [PubMed] [Google Scholar]