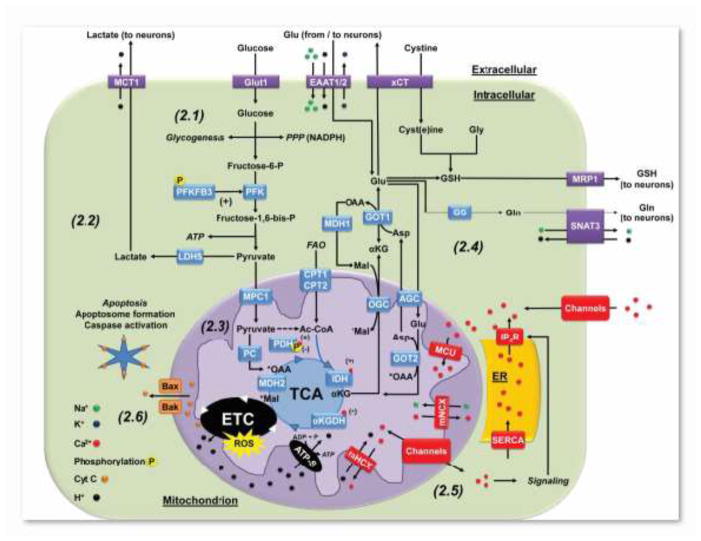
Figure 2.
Mitochondrial metabolism and signaling in astrocytes. 2.1: Glucose in astrocytes is used for glycogenesis, NADPH production through the PPP, or glycolysis. Astrocytes are highly glycolytic due to the expression of high levels of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-3 (PFKFB3), whose byproduct fructose-2,6-bisphosphate (F2,6P2), is a positive effector of the glycolytic enzyme 6-phosphofructo-1-kinase (PFK1). In addition, the activity of PFKFB3 is increased by phosphorylation by 5′-AMP-activated protein kinase (AMPK) (Bolanos 2016). 2.2: Astrocytes primarily derive ATP from glycolysis rather than oxidative phosphorylation, where pyruvate is converted to lactate by LDH5 and exported to the extracellular space to be consumed by neurons. 2.3: Astrocytes carboxylate pyruvate to oxaloacetate (OAA) via pyruvate carboxylase (PC) to regenerate TCA cycle intermediates. Phosphorylation of pyruvate dehydrogenase (PDH) restricts the conversion of pyruvate to acetyl-CoA (Ac-CoA). Thus, FAO has been proposed to be the primary contributor of Ac-CoA to the TCA cycle. 2.4: Alpha ketoglutarate (αKG) generated from the TCA cycle can be transported to the cytosol and converted to Glu by glutamic-oxaloacetic transaminase 1 or aspartate (Asp) aminotransferase (GOT1) as part of the malate-Asp shuttle. Glu has three central metabolic pathways in astrocytes. 1) Glu can be converted to Gln by GS and exported to neurons by the sodium-coupled neutral amino acid transporter 3 (SNAT3). 2) Glu is exchanged via xCT for extracellular cystine that is reduced to Cys. Extracellular Glu can be uptaken back by astrocytes by EAAT1/2. Finally, 3) Glu, Gly and Cys are precursors of GSH, which is also exported to neurons via MRP1. 2.5: The ER acts as a store for intracellular calcium, where the sarco/endoplasmic reticulum calcium ion ATPase (SERCA) pumps cytosolic Ca2+ into the ER. Ca2+ signaling is tightly regulated by the activation of IP3R that release Ca2+ from ER stores, as well as by the activation of plasma membrane Ca2+ channels. Mitochondria can buffer Ca2+ by its transport across the inner mitochondrial membrane to the matrix viaMCU), while the export is performed by mNCX and mHCX. Mitochondria can also transport Ca2+ in and out of the mitochondria via the activation of distinct Ca2+ permeable channels. In the matrix, Ca2+ stimulates TCA carbon flux by binding to PDH, IDH, and αKGDH, increasing the activity of the ETC and ATP production. 2.6: Cyt C is held close to the inner mitochondrial membrane by cardiolipin (not shown), acting as a component of ETC. Dissociation of Cyt C from cardiolipin, through oxidative or enzymatic means, coupled with permeabilization of the outer mitochondrial membrane by the formation of Bax/Bak oligomeric channels, allows Cyt C to escape into the cytosol. Cytosolic Cyt C associates with apoptotic protease-activating factor 1 (APAF1), forming the apoptosome and leading to the activation of caspases to initiate apoptosis. AGC, aspartate-glutamate carrier; CPT1 or 2, carnitine palmitoyltransferase isoform 1 or 2; MDH1 or 2, malate dehydrogenase isoform 1 or 2; MPC1, mitochondrial pyruvate carrier 1; OGC, 2-oxoglutarate (α-ketoglutarate) carrier.