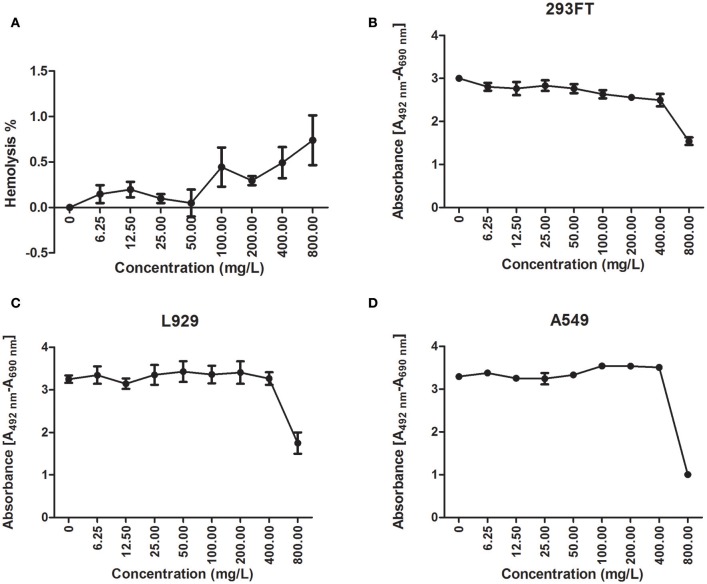
Figure 2.
Cytotoxicity of cathelicidin-BF. Hemolysis and cell viability assays were conducted to test the cytotoxicity of cathelicidin-BF to mammalian cells. (A) For the hemolysis assay, cathelicidin-BF dissolved in 0.9% saline was added to mouse erythrocytes diluted in 0.9% saline and incubated at 37°C for 30 min. Supernatants were collected by centrifugation at 1,000 × g for 15 min and further diluted four times with 0.9% saline to test the absorbance at 540 nm. Using 1% Triton X-100 (v/v) to determine 100% hemolysis and 0.9% saline as the negative control, the hemolysis rate of cathelicidin-BF is expressed as [(Absorbance sample-Absorbance control)/(Absorbance100%-Absorbance control)]×100. The cell proliferation kit II (XTT) (Roche) was used to test the effects of cathelicidin-BF on the viability of (B) 293FT (human embryonic kidney cells), (C) L929 (mice fibroblast cell line) and (D) A549 (adenocarcinomic human alveolar basal epithelial cells) cells. Absorbance [A492 nm-A690 nm] was used to quantify viable cells.