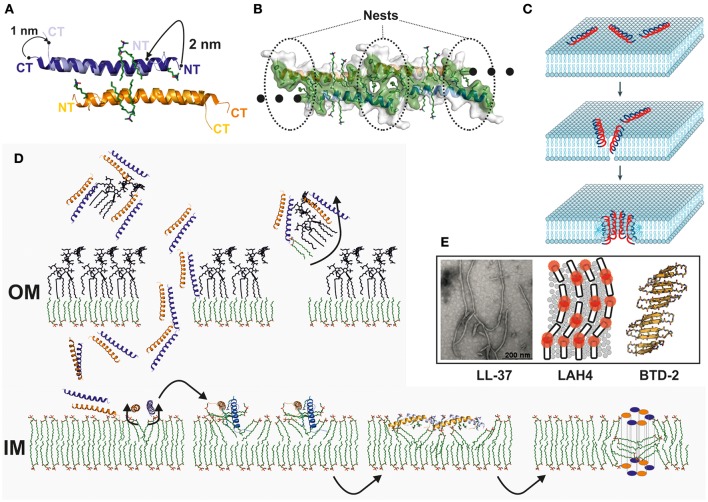
Figure 2.
Structure and membrane interaction mechanism of human cathelicidin. (A) Structure comparison of the LL-37 dimer in the presence and absence of detergents. Detergents induce a significant conformational change at the N- and C-terminus and discrete detergent binding sites are formed. (B) LL-37 tetramer in a surface representation. Hydrophobic residues on the side are marked in green. At the interface between two dimers, nest-like hydrophobic structures are formed to accommodate detergents in vitro. Lipid molecules in vivo may occupy these detergent positions, and lipid molecules or detergents may initiate the oligomerization of the channel. (C) Simplified mechanism describing the toroidal model which explain the activity of AMPs which are in a first step electrostatically attracted by membranes followed by their assembly and partial integration. In a final step the peptides form channels based on peptide-peptide and peptide-lipid interactions after full integration into lipid bilayers (Brogden, 2005). This figure is reprinted with permission from by Brogden (2005). (D) Model for the interaction of LL-37 with the cell wall of a Gram-negative bacterium. Significant interactions between LL-37 and LPS have been demonstrated, and, as a hypothesis, LPS may be translocated apart from the cell wall in order to build holes for the translocation of LL-37 into the periplasmic space (Scientific reports in press); Vandamme et al., 2012). Interactions of the peptide with lipid molecules will initiate the conformational changes, and fiber-like oligomers may form on the inner membrane. These fibers lead to an increased local concentration of the peptide and will interfere with the membrane stability. (E) Related models and experimental data which were recently published in the literature are based on fluorescence techniques applied to LAH4, crystallography and analysis of crystal packing of BTD-2 and electron microscopy of LL-37 mixed with lipid vesicles (Aisenbrey and Bechinger, 2014; Shahmiri et al., 2016; Wang et al., 2016). LL-37 TEM figure is reprinted from Shahmiri et al. (2016) published in open-access under CC BY 4.0 license. LAH4 figure is reprinted with permission from by Aisenbrey and Bechinger (2014). Copyright 2014 American Chemical Society. BTD-2 figure is reprinted with permission from Wang et al. (2016). Copyright 2016 American Chemical Society.