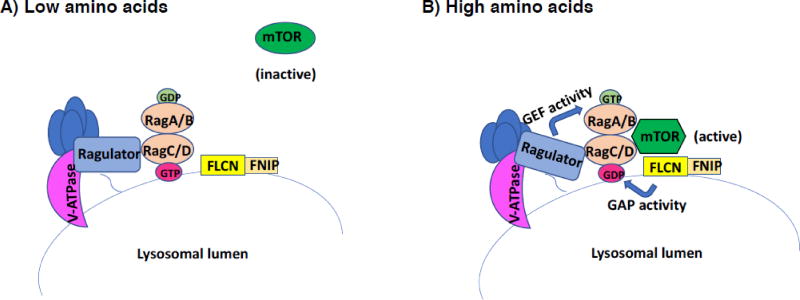
Figure 4.
FLCN acts as a GAP for RagC/D to activate mTOR on the lysosome surface in response to amino acids. mTORC1 activation by amino acids requires a lysosome-associated complex of proteins that include the vacuolar (v)-ATPase, the Ragulator complex, and Rag GTPases, which function as obligate heterodimers RagA or B and RagC or D. Amino acids signal from within the lysosomal lumen to Ragulator, which exerts its guanine nucleotide exchange factor (GEF) activity toward RagA/B. GTP-loaded RagA/B then recruits mTORC1 to the lysosomal surface for activation. Additionally, when amino acids are low, FLCN-FNIP associates with the lysosomal surface. When amino acid levels are sufficient, FLCN-FNIP acts as a GTPase activating protein (GAP) towards RagC/D. The GDP-loaded RagC/D can then facilitate mTORC1 recruitment to the lysosome for activation (Petit et al., 2013; Tsun et al., 2013).