Figure 3.
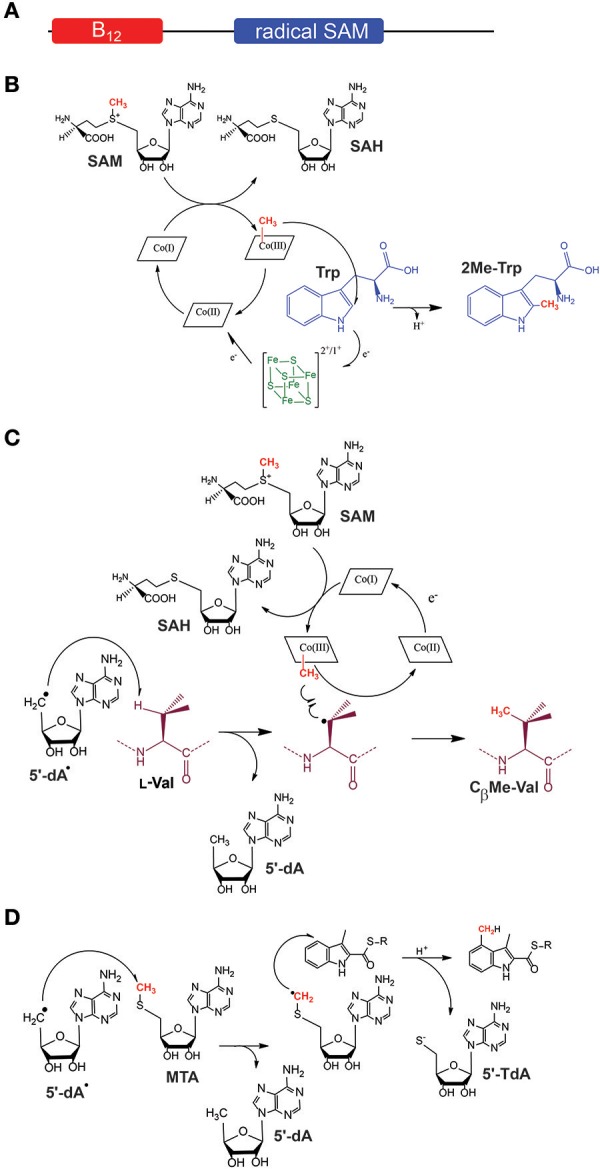
Proposed mechanisms for class B (B12-dependent) and class C radical SAM enzymes. (A) General architecture of B12-dependent radical SAM enzymes. (B) Proposed mechanism for TsrM, an sp2-hybridized carbon methyltransferase. (C) Proposed mechanism for PoyC, an sp3-hybridized carbon methyltransferase. In contrast to TsrM, PoyC catalyzes SAM homolytic cleavage likely to abstract a substrate Cβ H-atom. This intermediate is likely to react with a methyl radical species leading to the formation of Cβ methyl-valine. In both mechanisms, Cob(II)alamin is likely formed. After further reduction, Cob(I)alamin can react with SAM to regenerate methyl-cobalamin for the next catalytic cycle. (D) Proposed mechanism for NosN, a class C methyltransferase. NosN uses two molecules of SAM to generate 5′-dA radical and 5′-methylthioadenosine (MTA). The 5′-dA radical abstracts an H-atom from the methyl group of MTA leading to the addition of the radical intermediate to the C-4 of the indolyl substrate. After C-S bond cleavage, 5′-thioadenosine (5′-TdA) and the methylated indole product are released.
