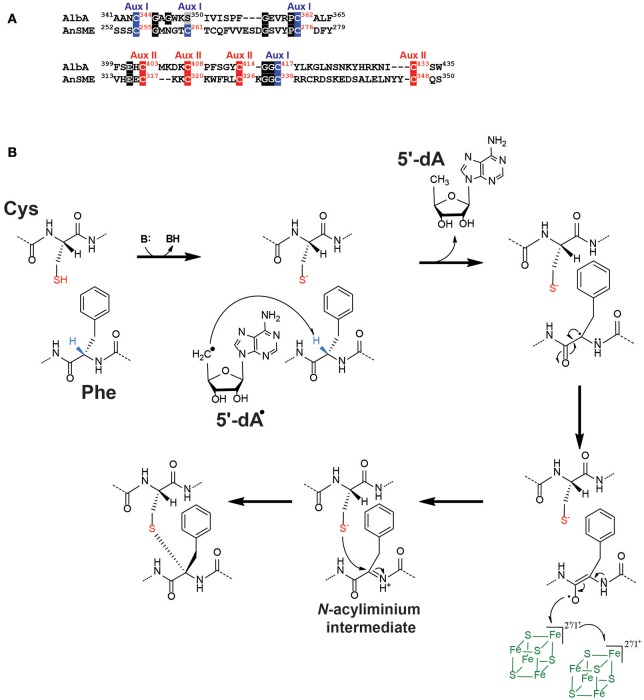
Figure 4.
Thioether bond formation by radical SAM enzymes. (A) Sequence alignment of AlbA and anSME showing the cysteine residues involved in the coordination of the [4Fe-4S] clusters present in the SPASM-domain. Cysteine residues involved in the auxiliary cluster I (Aux I) and auxiliary cluster II (Aux II) are indicated in blue and red, respectively. Numbers indicate the respective positions of the cysteines in the protein sequences of AlbA and anSME. (B) Proposed mechanism for AlbA. AlbA catalyzes H-atom abstraction on Cα-atom. The carbon-centered radical rearranges leading to the formation of an N-acyliminium intermediate. This intermediate is quenched by the thiolate group of a cysteine residue resulting in the formation of a Cα-thioether bond. The auxiliary clusters I and II are proposed to serve as an electron conduit.