Abstract
Mycobacterium tuberculosis, the pathogen that causes tuberculosis (TB), is becoming increasingly recognized as an important cause of fatal chronic illnesses in China. In this study, we report an infectious disease among 84 rhesus macaques at a Chinese zoo. Their clinical signs and symptoms were very similar with the manifestations of TB in humans. To determine the potential pathogens of this outbreak, many methods were used. First, tuberculin skin tests showed that none of the monkeys displayed significant skin reactions. Subsequently, the sera were tested for specific antibody IgG; 29 (34.5%) and 39 (46.4%) blood samples tested positive by TB-IgG and TB-DOT, respectively. Radiographic examination showed characteristic imageology changes in 14 (16.7%) monkeys. One individual determined as positive by the above three methods was euthanized, and histopathological analysis demonstrated typical granulomas and caseous necrosis in the lung, liver, spleen, and intestine. Furthermore, the pathogenic mycobacteria were isolated from lung lobe, cultured on acidic Lowenstein-Jensen culture medium, and identified as M. tuberculosis by real-time PCR and DNA sequencing. Nevertheless, the origin of the infection remained unknown. These findings emphasize the need to strengthen the management and training of staff, especially those working at animal shelters.
Keywords: infectious disease, Mycobacterium tuberculosis, outbreak, rhesus macaque
Introduction
Recently, a severe outbreak of an infectious disease occurred among 84 rhesus macaques in a zoo in China. The rhesus macaques have been quarantined to observe clinical signs, such as weight loss, coughing, and anorexia, which were very similar to the manifestations of tuberculosis (TB) in humans. TB, one of the major human infectious diseases, has been considered as a severe and even lethal disease responsible for 1.5 million deaths each year worldwide [42]. TB is caused by Mycobacterium tuberculosis, usually via the respiratory route within and between species, leading to serious pulmonary lesions accompanied by caseous necrosis and granuloma, as well as in other organs [39]. As an anthropozoonosis, TB has also been regarded as a serious infectious threat that could result in progressive pulmonary disease in many non-human primate (NHP) species. Indeed, among these, the rhesus macaques are highly susceptible to M. tuberculosis [17, 24]. Several outbreaks of M. tuberculosis infection in rhesus macaque have been previously reported [8, 11, 25, 33, 34, 38, 41, 43, 49]. Given that the infected animals could transmit the disease to humans, it becomes vital to identify the original pathogen and ways to control TB transmission between humans and animals.
Currently, accurate diagnosis of M. tuberculosis infection in NHP colonies remains challenging, and the infection may often go undetected for weeks or even months. The tuberculin skin test (TST) is well known as the gold standard screening tool for the diagnosis of M. tuberculosis infection in humans and NHP colonies. However, TST remains inadequate due to false-positive or false-negative results at times [2, 7, 27], and to its sensitivity and specificity [6, 10, 12, 19]. The false-positive results are due to cross reactivity of the tested samples with environmental non-tuberculous mycobacteria (NTM) or bacille Calmette-Guerin (BCG), while the false-negative results are known to occur because of anergic humans and animals with an inadequate T-cell response when severely infected with M. tuberculosis [2, 7, 10]. Therefore, other screening tools combining findings of serological, radiographical, pathological, microbiological, and cytological nature, with mycobacterial culture and qRT-PCR assays, should be used together with TST to make a definitive diagnosis of M. tuberculosis in NHP colonies.
The aim of this report was to determine the pathogen responsible for this outbreak by multiple screening assays and to call for adopting strict quarantine measures and conventional anti-tuberculosis antibody detection in NHP for optimal TB detection.
Materials and Methods
Animals and ethics statement
All 84 rhesus macaques suspected of contracting an infection of M. tuberculosis in a wild zoo in China were numbered randomly. These macaques ranging in age from 0.5 to 8 years and weighing 1.6–9.7 kg were observed at the zoo in 2013. Prior to TST and other examinations, the animals were anesthetized with 10 mg/kg ketamine hydrochloride. All animal experiments were approved by the Animal Ethical Committee, and the animal care met the committee’s standards. Rhesus macaques were well cared for in the facilities, and all efforts were made to minimize suffering according to Experimental Animal Regulation Ordinances defined by China National Science and Technology Commission.
Tuberculin skin test
The eyelid of rhesus macaque was used to observe the results of TST. In order to evaluate the results objectively and realistically, two methods [Purified protein derivative of tuberculin (TB-PPD) and recombinant CFP10/ESAT6 fusion protein] were used. The TST was performed by intradermal injection of 0.1 ml of TB-PPD (50 IU/ml, Sanroad Biological Products Co., Ltd., Beijing, China) into the left eyelid and 1 µg recombinant CFP10/ESAT6 fusion protein (Hisun Pharmaceutical Co., Ltd., Taizhou, Zhejiang, China) into the right eyelid. Eyelid reactions were recorded and graded with the standard 5-point scoring system based on erythema, bruising, and swelling at 24, 48, and 72 h after injection [7].
Detection of sera antibodies
Blood samples (5 ml) were collected from 84 rhesus macaques via femoral veins prior to TST, and then centrifuged at 3,000 rpm for 5 min to obtain the sera. The sera were stored at −20°C until use. Two commercial rapid colloidal gold immunoassay kits (qualitative analysis), TB-DOT (Upper Bio-Tech Pharma Co., Ltd., Shanghai, China) and TB-IgG (Modern Gaoda Biotechnology Co., Ltd., Beijing, China), were used to detect the M. tuberculosis specific IgG level according to the instructions of manufacturers. Additionally, sensitivity between the two assays was also compared. Each tested sample was read and recorded in 15–20 min, and the determination of results was based on color intensity of spot or line. Usually, it is considered as positive if the test spot or line is clear, and negative if not.
Chest X-ray (CXR) examination
Each rhesus macaque was gently fixed on the operating table by its arms and legs. The radiographs were obtained from a mobile radiographic unit (M226668CE) provided by the People’s Hospital of Dongsheng county (Inner Mongolia Autonomous Region, China) to diagnose the lung condition.
Necropsy
To identify any further gross lesions in the rhesus macaques, necropsy was performed, and all necessary safety precautions were taken by the pathologist during the procedure. One of the rhesus macaques which indicated positive results in sera antibodies and radiographs as well as clinical symptoms was selected to be euthanized via intravenous overdose injection of sodium pentobarbital prior. The chest and abdominal cavity were opened using a sterile scalpel, and the lungs, liver, spleen, and part of the intestines were removed. Subsequently, these organs were placed in a sterile container to observe their pathological status, and gross lesions were photographed in detail.
Histopathological examinations
The lungs, liver, spleen, and intestines were fixed in 10% (vol/vol) formalin overnight before paraffin embedding according to conventional methods. The organs were cut longitudinally across the coronal plane into 5-µm sections and stained with hematoxylin and eosin (H&E) for histopathological evaluation or Ziehl-Neelsen for acid-fast bacilli under an Olympus DP71 microscope.
Mycobacterial culture
Eighty-four rhesus macaques were divided into eight groups according to the results of TB-DOT, TB-IgG, and chest X-ray. The positive or negative samples in TB-DOT, TB-IgG, and chest X-ray examination were marked with D+/−, I+/−, and X+/−, respectively. One sample of lungs collected from a rhesus macaque in the D+I+X+ group was homogenized by a sterile grinder with equivalent volume of 1% sodium hydroxide. Subsequently, the lung homogenate was plated on acidic Lowenstein-Jensen culture medium (Baso Biotechnology Co., Ltd., Zhuhai, China) and incubated at 37°C for 4 weeks.
qRT-PCR and DNA sequencing
After the paraffin sections of the granulomas were cultured with Lowenstein-Jensen culture medium, the colonies were collected with a sterile tube and were heat inactivated (95°C, 30 min). Genomic DNA was extracted from the colonies, and strain-specific DNA fragment was amplified by qRT-PCR according to our previous study [46]. In addition, 16S rRNA gene fragment was amplified and sequenced by Sangon (Beijing, China) to confirm the qRT-PCR results [44]. Furthermore, the sequence was compared with those registered in the GenBank database by BLAST analyses. Only 100% identities with mycobacteria were determined as mycobacterium species.
Results
Eyelid reactions of rhesus macaques
TST were carried out in all 84 rhesus macaques by intradermal injection of TB-PPD (0.1 ml, 5 IU) into the left eyelid and recombinant CFP10/ESAT6 fusion protein (1 µg) into the right eyelid. The results showed that, regardless of the method used to perform TST, no significant swelling and nodules were observed on the left or right eyelid of experimental animals 24, 48, and 72 h after injection (Fig. 1).
Fig. 1.

Tuberculin skin test in M. tuberculosis-infected representative rhesus macaque. The rhesus macaques were intradermally injected in the left eyelid with 0.1 ml of 5 IU TB-PPD and in the right eyelid with 1 µg recombinant CFP10/ESAT6 fusion protein, respectively. No significant swelling and nodules were observed on the left or right eyelid of experimental animals at 24, 48, and 72 h after injection.
Serological and imageological analyses
Eighty-four rhesus macaques were tested for specific antibodies with TB-DOT (abbreviated as D in Table 1) and TB-IgG (abbreviated as I in Table 1), and examined lungs lesions by CXR (abbreviated as X in Table 1). As showed in Table 1, 29 (34.5%, D+I+/−X+/−), 39 (46.4%, D+/−I+X+/−), and 14 (16.7%, D+/−I+/−X+) positive cases were found with TB-DOT kit, TB-IgG kit, and chest X-Ray, respectively. Among those, 12 positive cases (14.3%, D+I+X−) were simultaneously found with both TB-DOT kit and TB-IgG kit, only one positive case (1.2%, D+I−X+) was simultaneously found with both TB-DOT kit and CXR, two positive cases (2.4%, D−I+X+) were simultaneously found with both TB-IgG kit and CXR, and six positive cases (7.1%, D+I+X+) were simultaneously found with all three methods.
Table 1. Screening of positive rhesus macaque cases.
| Da | Ia | Xb | Number |
|---|---|---|---|
| − | − | − | 29 |
| + | − | − | 10 |
| − | + | − | 19 |
| + | + | − | 12 |
| − | − | + | 5 |
| + | − | + | 1 |
| − | + | + | 2 |
| + | + | + | 6 |
| Total | 84 | ||
a: Blood samples were collected from rhesus macaques numbered randomly from 1 to 84, and the specific antibodies were detected with TB-DOT (abbreviated as D) and TB-IgG (abbreviated as I). The positive or negative samples in TB-DOT and TB-IgG examination were marked with D+/− and I+/−, respectively. b: The condition of lung was examined with chest X-ray (abbreviated as X). The positive or negative samples in chest X-ray examination were marked with X+ or X−.
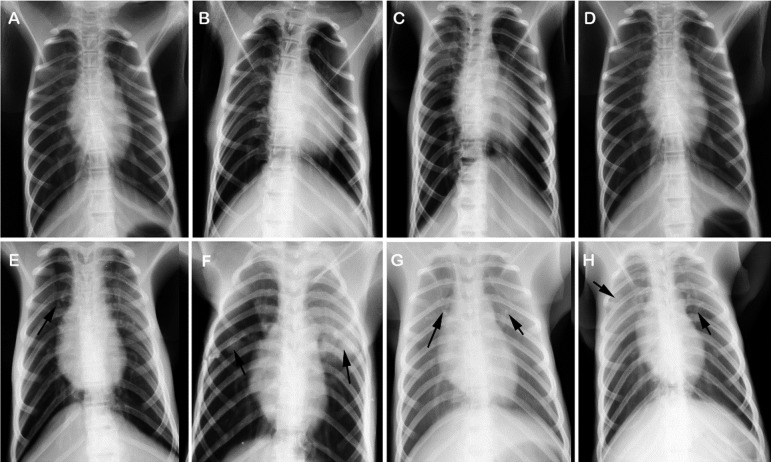
Moreover, the CXR examination showed that there was no obvious imageological lesion in the lungs from rhesus macaques in D+/−I+/−X−group (Figs. 2A–D). In contrast, flocculent shadows, tuberculoma, calcifications, and uneven distribution of tubercles were observed in the lungs from rhesus macaques in D+/−I+/−X+ group (Figs. 2E–H).
Fig. 2.
Chest X-ray of representative rhesus macaques in each group. Eight representative rhesus macaques (No. 2, 5, 11, 31, 77, 47, 70 and 53) in D−I−X−, D+I−X−, D−I+X−, D+I+X−, D−I−X+, D+I−X+, D−I+X+, and D+I+X+ group were chosen to evaluate the condition of lung, respectively. Their radiographs are indicated with A, B, C, D, E, F, G, and H in this figure. No obvious radiographic lesion was observed in the lung of rhesus macaques from D+/−I+/−X− groups. However, kinds of radiographic lesions were observed in the lung of rhesus macaques from D+/−I+/−X+ groups, including flocculent shadows, tuberculoma, calcifications, and uneven distribution of tubercles (indicated by arrow in E to H).
Necropsy and histopathological analyses
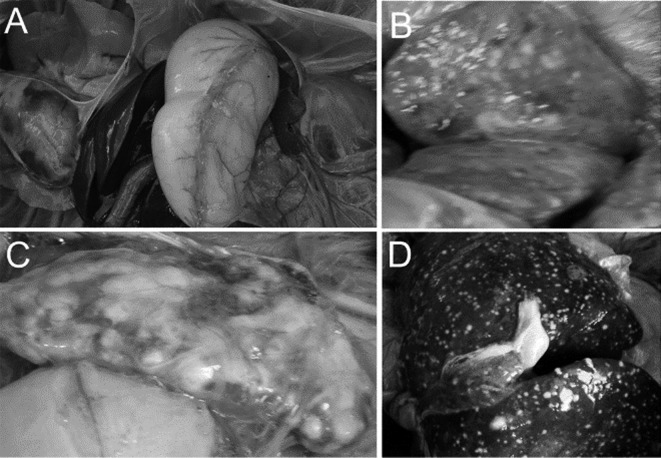
One rhesus macaque from each of the D−I−X− and D+I+X+ groups were euthanized via intravenous injection with overdose of sodium pentobarbital prior. Based on the necropsy, there were no distinct lesions in the lungs, spleen, and liver from rhesus macaque in D−I−X− group (Fig. 3A). In contrast, multiple miliary tubercles and caseous necrosis were observed in the lungs (Fig. 3B), spleen (Fig. 3C), and liver (Fig. 3D) of rhesus macaque in D+I+X+ group.
Fig. 3.
Gross pathological changes of organs collected from rhesus macaques infected with M. tuberculosis. One rhesus macaque each in D+I+X+ and D−I−X− (as a negative control) group was euthanized via intravenous injection with overdose of pentobarbital sodium. Vivisection and tissue dissection revealed no obvious pathological changes in the main visceral organs of the rhesus macaque in D−I−X− group (A). In contrast, numerous miliary tubercles and extensive caseous necrosis were observed on the surface of the lung (B), spleen (C), and liver (D) collected from the rhesus macaque in D+I+X+ group.
The histopathological tissue analysis revealed severe lesions in rhesus macaques in D+I+X+ group. Multiple areas of caseous necrosis of different sizes and inflammatory cell infiltration were observed in the lungs (Fig. 4A). The structure of the spleen was obviously damaged with multiple large granulomas, localized calcifications, and proliferation of connective tissue (Fig. 4B). Large areas of caseous necrosis surrounded by epithelioid cells and lymphocytes, inflammatory cells infiltration, and vacuolar degeneration were observed in the liver (Fig. 4C). In intestines, typical caseous necrosis was surrounded by fibrillar connective tissue and inflammatory cells (Fig. 4D). Additionally, acid-fast bacilli were observed in tissue sections of lung by acid-fast stain (Fig. 4E).
Fig. 4.
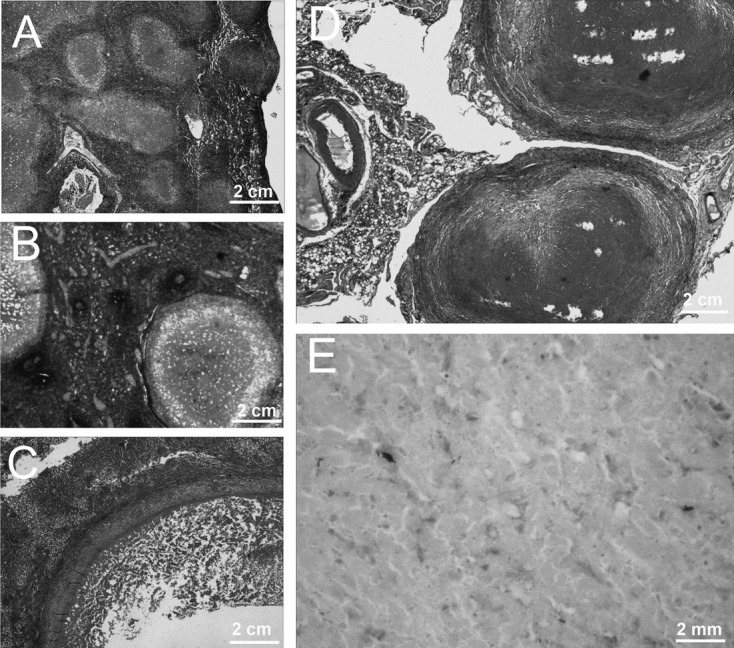
Histopathological analyses. The organs collected from the rhesus macaque in D+I+X+ group were subjected to histopathological examination. Large caseous granuloma and infiltration of inflammatory cells in lung (A), localized calcification, proliferation of connective tissue, and caseous necrosis in spleen (B), large caseous granuloma with central necrosis surrounded by a narrow mantle zone in the liver (C), inflammatory cell infiltration, caseous necrosis, and proliferation of connective tissue in intestines (D) were observed by H&E stain using light microscopy at a magnification of 100×. Additionally, acid-fast bacilli were observed in a tissue section of lung by acid-fast stain (E) under light microscope at original magnification times 1,000×.
Mycobacterial culture and species identification
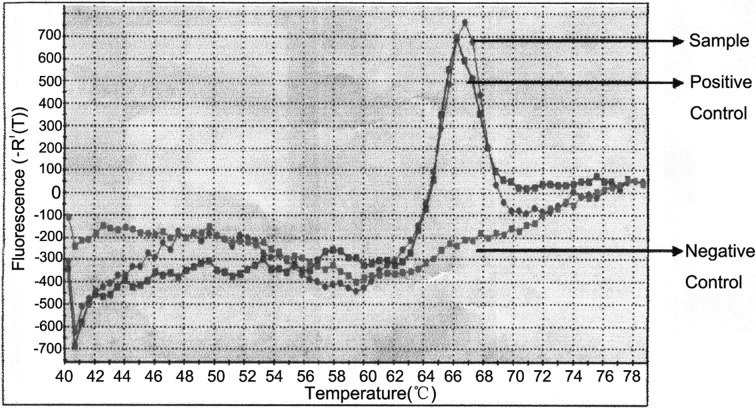
In order to determine the pathogen, lung samples collected from rhesus macaque in D+I+X+ group were cultured after grinding. After 4 weeks, cauliflower-like colonies were observed on the Lowenstein-Jensen culture medium. A fraction of bacterial colony was used to qRT-PCR assay and DNA sequencing. The results confirmed the presence of M. tuberculosis complex-specific DNA in the colony (Fig. 5), which was further confirmed by the presence of M. tuberculosis 16S rRNA gene by sequencing analysis (100% similarity).
Fig. 5.
qRT-PCR analysis. Genomic DNA was extracted from the colony cultured from the lung tissue of the rhesus macaque in D+I+X+ group and amplified using qRT-PCR kit according to the manufacturer’s instructions. The fluorescence intensity of the sample (gray), positive control (red), and negative control (green) was detected with 7,300 Real Time PCR System (Applied Biosystems).
Discussion
M. tuberculosis remains a worldwide major cause of infectious disease-related mortality. Although M. tuberculosis infection has merited heightened awareness, recognition, and frequently appropriate treatment, fatality of TB remains high in humans and domestic animals worldwide [20]. According to the World Health Organization (WHO) global TB report, as many as 9.6 million persons are infected by M. tuberculosis, and almost 1.5 million died from the disease in 2014 [42]. Similarly, a report presented by the National Bureau of Statistics (NBS) of China estimated almost 889,381 new TB cases and 2,240 TB deaths in 2014, which indicated that TB remains a serious health problem in China.
As an anthropozoonosis, TB can spread between humans and animals [14, 40]. It has been recently demonstrated that NHP were the most susceptible of all captive animals to tuberculosis [26]. The lung is the most easily infected organ in humans and NHP. Furthermore, the pulmonary manifestations caused by mycobacterium infections in NHP were similar to those observed in humans, and included caseous granulomata, calcification, tuberculoma, and cavity lesions [3, 47]. These indicate that the major modes of transmission of M. tuberculosis from infected humans to animals are coughing, sneezing, expectoration, or contaminated food [35, 40]. With the recent development of global economy and transportation, international travel and trade are developing rapidly in China, thus increasing the risk of TB transmission. Therefore, surveillance and response systems for anthropozoonosis must be strengthened in 22 high-burden TB countries that contribute as high as 80% of the global burden of TB, particularly India and China, which export large numbers of NHP [21, 42].
In addition to well-established systems, early diagnosis is a crucial process for the prevention and treatment of TB infection. To diagnose M. tuberculosis infection in a timely manner, several methods have been proposed and used in previous studies, such as mammalian old tuberculin (MOT) or TB-PPD, mycobacterial culture, serum antibody detection, pathological analysis, imageological examination, and PCR assays [1, 4, 28, 38, 42]. MOT has been the mainstay for screening M. tuberculosis infection in NHP colonies for more than 60 years. However, as we were unable to obtain commercial MOT kit in China, we opted to use commercially produced TB-PPD to perform TST in this study. As one of the methods used early in clinical diagnosis, TB-PPD is recommended by the American Thoracic Society and the Centers for Disease Control and Prevention [16]. In this study, none of the 84 rhesus macaques exhibited any reaction to TB-PPD and recombinant CFP10/ESAT6 fusion protein in eyelids after 72 h; the results suggested that rhesus macaques were not sensitive to M. tuberculosis antigen and required higher doses of antigen in skin tests compared with humans, which is consistent with a previous report [13]. Further studies are needed to determine the appropriate doses of antigens for inducing skin reaction in the rhesus macaques.
TB-PPD was considered as the standard method in the diagnosis of M. tuberculosis infection, but debates are ongoing regarding inadequacies in terms of both false-positive and false-negative results in assays [2, 7, 16]. Hence, other screening methods were used to make a definitive diagnosis of M. tuberculosis in this study. The lung is most easily infected by M. tuberculosis via droplets transmission. After infection, M. tuberculosis begins to grow in the lung following the decline of immunity of the rhesus macaques, which results in further damages in other organs [18]. In this study, typical caseous granulomas, composed of central caseation and surrounding infiltrated lymphocytes and proliferated connective tissue, were observed in the lungs, spleen, and liver by histopathology. Caseous granuloma is considered a key protection mechanism in the immunopathogenesis of TB infection, prevention, and treatment [9, 15]. Moreover, the acid-fast bacilli could be observed in the lung section, which indicates that the source of this outbreak was M. tuberculosis [9]. These findings were confirmed by CXR manifestations, such as calcification, tuberculoma, and ground-glass opacity. A previous study has advanced CXR as a screening tool in TB diagnosis, however, the sensitivity and specificity of the CXR were unsatisfactory [36], because the results depended on the doctor’s knowledge and experience. Therefore, it is necessary to improve the diagnostic performance by a well-defined scoring system, CXR quality control, and clinical manifestation.
Although mycobacterial culture is considered as the standard method for the diagnosis of TB [31, 42], mycobacterium is characterized by slow growth, with characteristic colonies usually requiring 3 to 4 weeks, which limits its application in public health emergencies. Compared to traditional mycobacterial culture, the qPCR assay was described as a specific and sensitive method to detect mycobacteria rapidly directly from clinical specimens [4, 29, 32, 45]. In our study, a lung section taken from infected rhesus macaques was ground and cultured on Lowenstein-Jensen culture medium. Four weeks later, cauliflower-like colonies were observed, which was similar to the colony characteristics of M. tuberculosis. Additionally, DNA sequencing using a small amount of the colony revealed that the pathogen in this case was M. tuberculosis. These findings corroborate our pathological results. Some studies indicated that PCR-based species identification is valuable for M. tuberculosis detection in dogs and emphasized that it is crucial to use such methods to diagnose TB in animals successfully [4, 14, 29, 37].
Among the diagnostic methods examined during the course of M. tuberculosis infection, serological diagnosis has advantages in its rapidity, sensitivity, reliability, noninvasiveness, convenience, and low cost [5, 22, 23, 30, 47]. In recent studies, TB-antibody, Luciferase Immunoprecipitation Systems (LIPS), TB related interferon-gamma release assay (TB-IGRA), and QuantiFERON-TB Gold In-Tube (QFT-GIT) were used to detect M. tuberculosis-specific antibodies in the sera or interferon gamma in whole blood samples from humans and rhesus monkeys. These methods had a higher diagnostic sensitivity and specificity compared with traditional methods [5, 22, 23, 30]. In this study, two different TB-antibody detection kits, TB-DOT and TB-IgG, were used to detect M. tuberculosis-specific IgG. As a result, at least 50 (59.5%) rhesus macaques were confirmed to be infected by M. tuberculosis; the sensitivity of TB-IgG (78%, 39/50) was higher than that of TB-DOT (58%, 29/50), which was consistent with a previous study [48].
In conclusion, we determined that the pathogen of this outbreak was M. tuberculosis, but the source was still unclear. We demonstrated that qPCR and serological methods are valuable and sensitive tools for normal or emergency screening in M. tuberculosis infection. Additionally, as an anthropozoonosis, TB can be transmitted between humans and animals, and a perfect management system, scientific warning system, emergency rescue system, and regular screening mechanism are very important for prevention of such transmission.
Conflict of Interest
All authors declare no commercial or other associations that might pose a conflict of interest.
Acknowledgments
This study was supported by the grant from the Army “Twelfth Five” Scientific Research Foundation (BWS11J050) and the Serious Infectious Diseases Special Foundation of China (2008ZX-10003–001), and Key Subject Foundation of the 309th Hospital of Chinese PLA (2016-ZD-001).
References
- 1.American Thoracic Society. 2000. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am. J. Respir. Crit. Care Med. 161: 1376–1395. [DOI] [PubMed] [Google Scholar]
- 2.Arend S.M., van Soolingen D., Ottenhoff T.H., van Dissel J.T.2001. Repeatedly negative tuberculin skin tests followed by active tuberculosis in an immunocompetent individual. Neth. J. Med. 58: 76–81. doi: 10.1016/S0300-2977(00)00100-5 [DOI] [PubMed] [Google Scholar]
- 3.Basaraba R.J.2008. Experimental tuberculosis: the role of comparative pathology in the discovery of improved tuberculosis treatment strategies. Tuberculosis (Edinb.) 88:(Suppl 1): S35–S47. doi: 10.1016/S1472-9792(08)70035-0 [DOI] [PubMed] [Google Scholar]
- 4.Bonovska M., Tzvetkov Y., Najdenski H., Bachvarova Y.2005. PCR for detection of Mycobacterium tuberculosis in experimentally infected dogs. J. Vet. Med. B Infect. Dis. Vet. Public Health 52: 165–170. doi: 10.1111/j.1439-0450.2005.00839.x [DOI] [PubMed] [Google Scholar]
- 5.Burbelo P.D., Keller J., Wagner J., Klimavicz J.S., Bayat A., Rhodes C.S., Diarra B., Chetchotisakd P., Suputtamongkol Y., Kiertiburanakul S., Holland S.M., Browne S.K., Siddiqui S., Kovacs J.A.2015. Serological diagnosis of pulmonary Mycobacterium tuberculosis infection by LIPS using a multiple antigen mixture. BMC Microbiol. 15: 205. doi: 10.1186/s12866-015-0545-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bushmitz M., Lecu A., Verreck F., Preussing E., Rensing S., Mätz-Rensing K., EPV-Tuberculosis Working Group on Non-human Primate Health.2009. Guidelines for the prevention and control of tuberculosis in non-human primates: recommendations of the European Primate Veterinary Association Working Group on Tuberculosis. J. Med. Primatol. 38: 59–69. doi: 10.1111/j.1600-0684.2008.00303.x [DOI] [PubMed] [Google Scholar]
- 7.Capuano S.V., 3rd, Croix D.A., Pawar S., Zinovik A., Myers A., Lin P.L., Bissel S., Fuhrman C., Klein E., Flynn J.L.2003. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect. Immun. 71: 5831–5844. doi: 10.1128/IAI.71.10.5831-5844.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corcoran K.D., Jaax G.P.1991. An attempt to predict anergy in tuberculosis suspect cynomolgus monkeys. Lab. Anim. Sci. 41: 57–62. [PubMed] [Google Scholar]
- 9.Ehlers S., Schaible U.E.2013. The granuloma in tuberculosis: dynamics of a host-pathogen collusion. Front. Immunol. 3: 411. doi: 10.3389/fimmu.2012.00411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farhat M., Greenaway C., Pai M., Menzies D.2006. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int. J. Tuberc. Lung Dis. 10: 1192–1204. [PubMed] [Google Scholar]
- 11.Garcia M.A., Bouley D.M., Larson M.J., Lifland B., Moorhead R., Simkins M.D., Borie D.C., Tolwani R., Otto G.2004. Outbreak of Mycobacterium bovis in a conditioned colony of rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis) macaques. Comp. Med. 54: 578–584. [PubMed] [Google Scholar]
- 12.Garcia M.A., Yee J., Bouley D.M., Moorhead R., Lerche N.W.2004. Diagnosis of tuberculosis in macaques, using whole-blood in vitro interferon-gamma (PRIMAGAM) testing. Comp. Med. 54: 86–92. [PubMed] [Google Scholar]
- 13.Good R.C., McCarroll N.E.1978. BCG Vaccination in Rhesus macaques: Study of Skin Hypersensitivity and Durationof Protective Immunity. Smithsonian Institution Press, Washington, DC. [Google Scholar]
- 14.Hackendahl N.C., Mawby D.I., Bemis D.A., Beazley S.L.2004. Putative transmission of Mycobacterium tuberculosis infection from a human to a dog. J. Am. Vet. Med. Assoc. 225: 1573–1577, 1548. doi: 10.2460/javma.2004.225.1573 [DOI] [PubMed] [Google Scholar]
- 15.Hunter R.L., Jagannath C., Actor J.K.2007. Pathology of postprimary tuberculosis in humans and mice: contradiction of long-held beliefs. Tuberculosis (Edinb.) 87: 267–278. doi: 10.1016/j.tube.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 16.Flynn J.P., Bentley S.E.1975. Topics in tuberculosis: diagnostic standards and classification of tuberculosis and other mycobacterial disease. Md. State Med. J. 24: 71–74. [PubMed] [Google Scholar]
- 17.Langermans J.A., Doherty T.M., Vervenne R.A., van der Laan T., Lyashchenko K., Greenwald R., Agger E.M., Aagaard C., Weiler H., van Soolingen D., Dalemans W., Thomas A.W., Andersen P.2005. Protection of macaques against Mycobacterium tuberculosis infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Vaccine 23: 2740–2750. doi: 10.1016/j.vaccine.2004.11.051 [DOI] [PubMed] [Google Scholar]
- 18.Lewis K.N., Liao R., Guinn K.M., Hickey M.J., Smith S., Behr M.A., Sherman D.R.2003. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guérin attenuation. J. Infect. Dis. 187: 117–123. doi: 10.1086/345862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin P.L., Yee J., Klein E., Lerche N.W.2008. Immunological concepts in tuberculosis diagnostics for non-human primates: a review. J. Med. Primatol. 37:(Suppl 1): 44–51. doi: 10.1111/j.1600-0684.2007.00261.x [DOI] [PubMed] [Google Scholar]
- 20.LoBue P.A., Enarson D.A., Thoen C.O.2010. Tuberculosis in humans and animals: an overview. Int. J. Tuberc. Lung Dis. 14: 1075–1078. [PubMed] [Google Scholar]
- 21.Mätz-Rensing K., Hartmann T., Wendel G.M., Frick J.S., Homolka S., Richter E., Munk M.H., Kaup F.J.2015. Outbreak of tuberculosis in a colony of rhesus monkeys (macaca mulatta) after possible indirect contact with a human TB patient. J. Comp. Pathol. 153: 81–91. doi: 10.1016/j.jcpa.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 22.Meng C., Wan T., Xu Z., Shan F., Fan F., Chen X., Jiao X.2015. [Immunogenicity evaluation of Mycobacterium tuberculosis MPT83 protein and establishment of serological diagnostic method for bovine tuberculosis detection]. Wei Sheng Wu Xue Bao 55: 220–226. (in Chinese) [PubMed] [Google Scholar]
- 23.Min F., Pan J., Wu R., Chen M., Kuang H., Zhao W.2016. Profiling serum antibodies to Mycobacterium tuberculosis proteins in rhesus monkeys with nontuberculous Mycobacteria. Exp. Anim. 65: 11–16. doi: 10.1538/expanim.15-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montali R.J., Mikota S.K., Cheng L.I.2001. Mycobacterium tuberculosis in zoo and wildlife species. Rev. Off. Int. Epizoot. 20: 291–303. doi: 10.20506/rst.20.1.1268 [DOI] [PubMed] [Google Scholar]
- 25.Murakami P.S., Monego F., Ho J.L., Gibson A., Javorouski M.L., Bonat M., Lacerda O., Brockelt S.R., Biesdorf S.M., Nakatani S.M., Riediger I.N., Fuverki R.B., Biava J.S., Vieira R.F., do Santos A.P., de Barros Filho I.R., Biondo A.W.2012. Detection of RD(Rio) strain of Mycobacterium tuberculosis in tapirs (Tapirus terrestris) from a zoo in Brazil. J. Zoo Wildl. Med. 43: 872–875. doi: 10.1638/2010-0108R.1 [DOI] [PubMed] [Google Scholar]
- 26.O’Reilly L.M., Daborn C.J.1995. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tuber. Lung Dis. 76:(Suppl 1): 1–46. doi: 10.1016/0962-8479(95)90591-X [DOI] [PubMed] [Google Scholar]
- 27.Oztürk N., Sürücüoğlu S., Ozkütük N., Gazi H., Akçali S., Köroğlu G., Ciçek C.2007. [Comparison of interferon-gamma whole blood assay with tuberculin skin test for the diagnosis of tuberculosis infection in tuberculosis contacts]. Mikrobiyol. Bul. 41: 193–202. (in Turkish) [PubMed] [Google Scholar]
- 28.Parsons S.D., Warren R.M., Ottenhoff T.H., Gey van Pittius N.C., van Helden P.D.2012. Detection of Mycobacterium tuberculosis infection in dogs in a high-risk setting. Res. Vet. Sci. 92: 414–419. doi: 10.1016/j.rvsc.2011.03.026 [DOI] [PubMed] [Google Scholar]
- 29.Posthaus H., Bodmer T., Alves L., Oevermann A., Schiller I., Rhodes S.G., Zimmerli S.2011. Accidental infection of veterinary personnel with Mycobacterium tuberculosis at necropsy: a case study. Vet. Microbiol. 149: 374–380. doi: 10.1016/j.vetmic.2010.11.027 [DOI] [PubMed] [Google Scholar]
- 30.Qian F., Wang W., Qiu Z., Shen Y., He J., Li D., Zhong J., Dai L.2013. Evaluation of a new tuberculosis-related interferon gamma release assay for tuberculosis infection diagnosis in Huzhou, eastern China. Indian J. Pathol. Microbiol. 56: 125–128. doi: 10.4103/0377-4929.118694 [DOI] [PubMed] [Google Scholar]
- 31.Ramsey I.K.2008. Infectious diseases of the dog and cat. J. Small Anim. Pract. 49: 488. doi: 10.1111/j.1748-5827.2008.00601.x [DOI] [Google Scholar]
- 32.Rocchetti T.T., Silbert S., Gostnell A., Kubasek C., Widen R.2016. Validation of a Multiplex Real-Time PCR Assay for detection of Mycobacterium spp., Mycobacterium tuberculosis complex, and Mycobacterium avium complex directly from clinical samples by use of the BD Max Open System. J. Clin. Microbiol. 54: 1644–1647. doi: 10.1128/JCM.00241-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schroeder C.R.1938. Acquired Tuberculosis in the Primate in Laboratories and Zoölogical Collections. Am. J. Public Health Nations Health 28: 469–475. doi: 10.2105/AJPH.28.4.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stetter M.D., Mikota S.K., Gutter A.F., Monterroso E.R., Dalovisio J.R., Degraw C., Farley T.1995. Epizootic of Mycobacterium bovis in a zoologic park. J. Am. Vet. Med. Assoc. 207: 1618–1621. [PubMed] [Google Scholar]
- 35.Toth A., Fackelmann J., Pigott W., Tolomeo O.2004. Tuberculosis prevention and treatment. Can. Nurse 100: 27–30. [PubMed] [Google Scholar]
- 36.van Cleeff M.R., Kivihya-Ndugga L.E., Meme H., Odhiambo J.A., Klatser P.R.2005. The role and performance of chest X-ray for the diagnosis of tuberculosis: a cost-effectiveness analysis in Nairobi, Kenya. BMC Infect. Dis. 5: 111. doi: 10.1186/1471-2334-5-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Soolingen D., Qian L., de Haas P.E., Douglas J.T., Traore H., Portaels F., Qing H.Z., Enkhsaikan D., Nymadawa P., van Embden J.D.1995. Predominance of a single genotype of Mycobacterium tuberculosis in countries of east Asia. J. Clin. Microbiol. 33: 3234–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vervenne R.A., Jones S.L., van Soolingen D., van der Laan T., Andersen P., Heidt P.J., Thomas A.W., Langermans J.A.2004. TB diagnosis in non-human primates: comparison of two interferon-gamma assays and the skin test for identification of Mycobacterium tuberculosis infection. Vet. Immunol. Immunopathol. 100: 61–71. doi: 10.1016/j.vetimm.2004.03.003 [DOI] [PubMed] [Google Scholar]
- 39.Via L.E., Lin P.L., Ray S.M., Carrillo J., Allen S.S., Eum S.Y., Taylor K., Klein E., Manjunatha U., Gonzales J., Lee E.G., Park S.K., Raleigh J.A., Cho S.N., McMurray D.N., Flynn J.L., Barry C.E., 3rd2008. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect. Immun. 76: 2333–2340. doi: 10.1128/IAI.01515-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viegas S.O., Ghebremichael S., Massawo L., Alberto M., Fernandes F.C., Monteiro E., Couvin D., Matavele J.M., Rastogi N., Correia-Neves M., Machado A., Carrilho C., Groenheit R., Källenius G., Koivula T.2015. Mycobacterium tuberculosis causing tuberculous lymphadenitis in Maputo, Mozambique. BMC Microbiol. 15: 268. doi: 10.1186/s12866-015-0603-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward G.S., Elwell M.R., Tingpalapong M., Pomsdhit J.1985. Use of streptomycin and isoniazid during a tuberculosis epizootic in a rhesus and cynomolgus breeding colony. Lab. Anim. Sci. 35: 395–399. [PubMed] [Google Scholar]
- 42.WHO. 2015. Global Tuberculosis Report-2015, Global Tuberculosis Report. World Health Organization, Geneva. [Google Scholar]
- 43.Wolf R.H., Gibson S.V., Watson E.A., Baskin G.B.1988. Multidrug chemotherapy of tuberculosis in rhesus monkeys. Lab. Anim. Sci. 38: 25–33. [PubMed] [Google Scholar]
- 44.Wu X., Zhang J., Liang J., Lu Y., Li H., Li C., Yue J., Zhang L., Liu Z.2007. Comparison of three methods for rapid identification of mycobacterial clinical isolates to the species level. J. Clin. Microbiol. 45: 1898–1903. doi: 10.1128/JCM.02253-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zak D.E., Penn-Nicholson A., Scriba T.J., Thompson E., Suliman S., Amon L.M., Mahomed H., Erasmus M., Whatney W., Hussey G.D., Abrahams D., Kafaar F., Hawkridge T., Verver S., Hughes E.J., Ota M., Sutherland J., Howe R., Dockrell H.M., Boom W.H., Thiel B., Ottenhoff T.H., Mayanja-Kizza H., Crampin A.C., Downing K., Hatherill M., Valvo J., Shankar S., Parida S.K., Kaufmann S.H., Walzl G., Aderem A., Hanekom W.A., ACS and GC6-74 cohort study groups.2016. A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet 387: 2312–2322. doi: 10.1016/S0140-6736(15)01316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J., Wang J., Sun W., Liang Y., Yang Y., Wang L., Yin M., Wu X.2016. Development and preliminary evaluation on rapid identification of Mycobacterium species by fluorescence quantitative PCR-probe melting curve method. Chin. J. Med. 51: 82–86. [Google Scholar]
- 47.Zhang J., Xian Q., Guo M., Huang Z., Rao Y., Wang Y., Wang X., Bao R., Evans T.G., Hokey D., Sizemore D., Ho W.Z.2014. Mycobacterium tuberculosis Erdman infection of rhesus macaques of Chinese origin. Tuberculosis (Edinb.) 94: 634–643. doi: 10.1016/j.tube.2014.08.005 [DOI] [PubMed] [Google Scholar]
- 48.Zhou W.M., Wang C.X.2011. Analysis on the detection results of 3 pulmonary tuberculosis antibodies in the serum of 413 tuberculosis patients. Prev. Med. Tribune 17: 70–71. [Google Scholar]
- 49.Zumpe D., Silberman M.S., Michael R.P.1980. Unusual outbreak of tuberculosis due to Mycobacterium bovis in a closed colony of rhesus monkeys (Macaca mulatta). Lab. Anim. Sci. 30: 237–240. [PubMed] [Google Scholar]