Figure 4. Model of brown adipocyte pathways regulated by mTORC2.
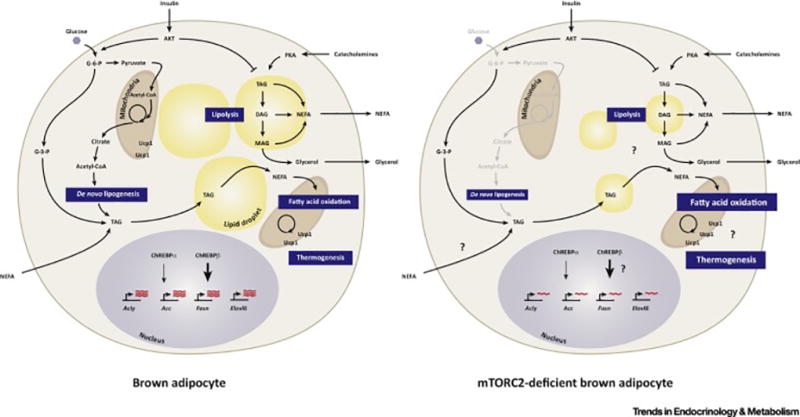
(Left) Brown adipocytes are specialized for adaptive thermogenesis, which is mediated by their unique expression of the mitochondrial localized uncoupling protein 1 (Ucp1) protein. Compared to white adipocytes, active brown adipocytes characteristically have multiple smaller lipid droplets (i.e. multi-locular), more mitochondria, increased glucose and lipid uptake, increased lipolysis and lipogenesis, and elevated fatty acid oxidation.
(Right) Mice in which the mTORC2 subunit Rictor is conditionally deleted in Myf5+ precursor cells (e.g. with Myf5-Cre), which give rise to the major brown fat depots in mice, results in decreased BAT size, decreased markers of lipogenesis, smaller lipid droplets, and increased characteristics of fatty acid oxidation and thermogenesis. This suggests mTORC2 loss in BAT may reprogram BAT metabolism in favor of energy expenditure over energy storage. However, Myf5+ precursors also give rise to skeletal muscle cells and many other non-brown adipocyte cell types, and moreover, Myf5-Cre deletes Rictor early in development prior to BAT specification, so many questions remain as to the specificity of this phenotype and the role that BAT mTORC2 plays in glucose and lipid uptake, ChREBP activity, lipolysis, and UCP1-mediated thermogenesis.
