Figure 7.
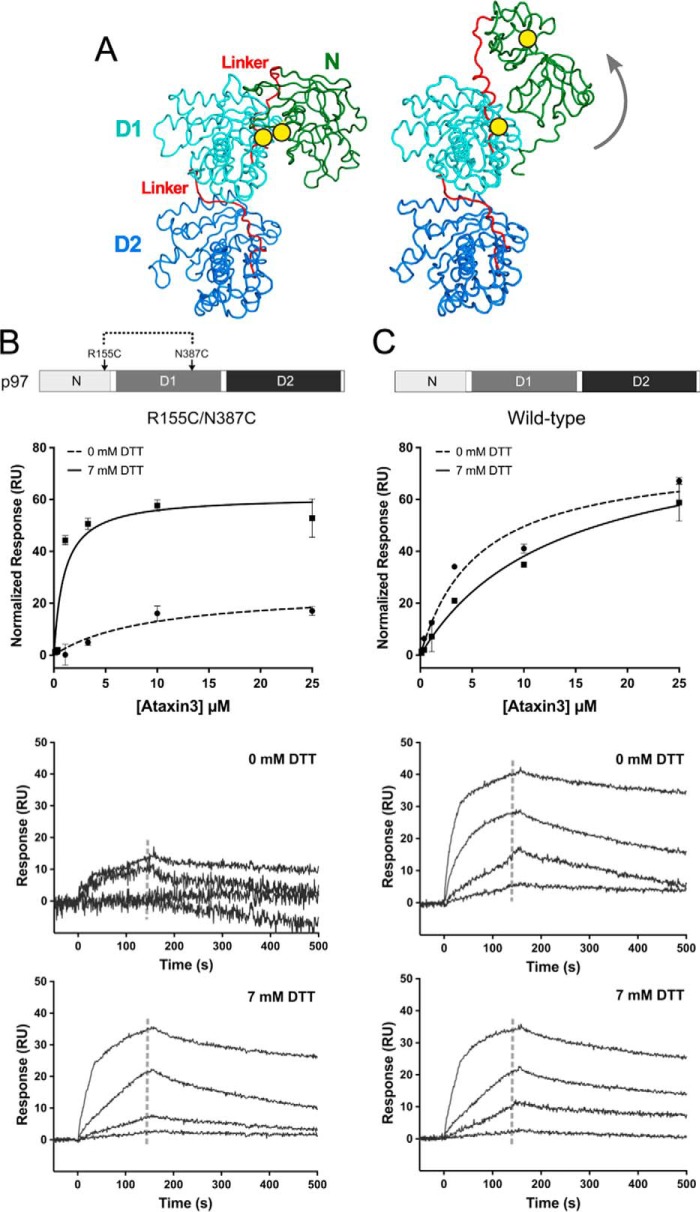
Conformationally-locked form of p97 cannot bind ataxin3. A, ribbon representations of the side view of a p97 protomer (PDB entries left, 5FTK and right, 5FTM), with the N-, D1-, and D2-domains shown in green, cyan, and deep blue respectively, and the two linkers in red. The yellow circles mark the positions of the R155C and N387C mutations that form a disulfide bond under oxidizing conditions (− DTT), locking the N-domain in the down state (left). When the disulfide bond is reduced (+ DTT), the N-domain is flexible and free to move to the up state (right), as indicated by the gray arrow. B and C, top panels show normalized SPR equilibrium responses fit to one-site binding models, and bottom two panels show binding to immobilized p97 with and without 7 mm DTT. B, R155C/N387C double mutant; C, wild-type p97 (n ≥ 3 for each concentration). The gray dashed lines in the sensorgrams represent the response range used to determine the fit.