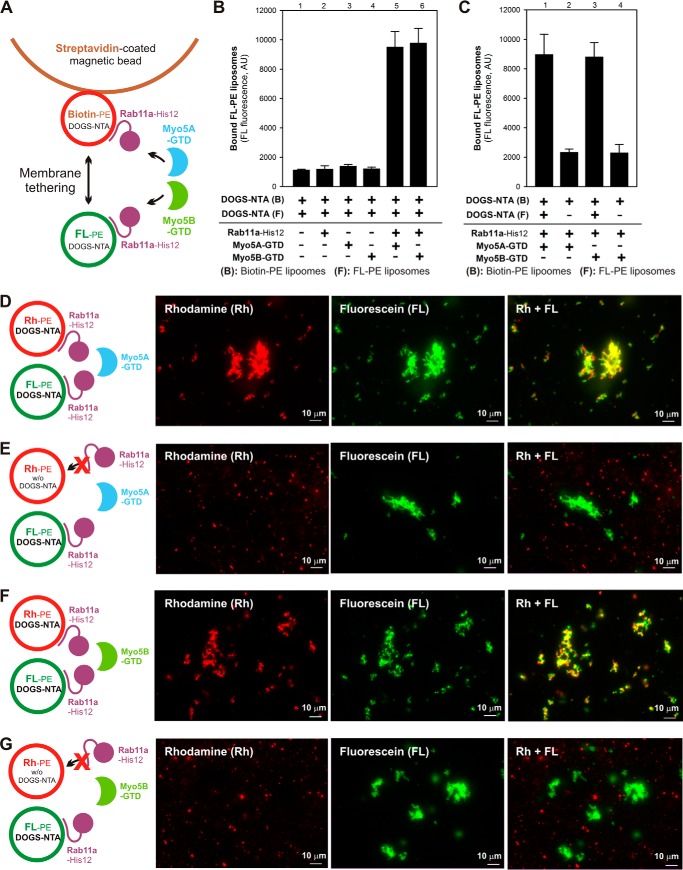
Figure 11.
Rab11a- and Myo5-GTD–dependent membrane tethering requires the membrane attachment of Rab11a on both of two opposing membranes destined to tether. A, schematic representation of the streptavidin bead–based liposome-tethering assay described in B and C using two types of liposomes bearing either biotin-PE/DOGS-NTA/Rh-PE or FL-PE/DOGS-NTA and purified Rab11a-His12 and Myo5-GTD proteins. B, streptavidin bead–based liposome-tethering assays with Rab11a-His12 and Myo5-GTDs. The biotin-labeled and FL-labeled liposomes were incubated with streptavidin-coated magnetic beads (30 °C, 2 h), mixed with Rab11a-His12 and Myo5A-GTD or Myo5B-GTD, and incubated further (30 °C, 10 min). The FL-labeled liposomes tethered to the biotin-labeled liposomes on streptavidin beads were quantified by measuring the FL fluorescence. For a control, Rab11a, Myo5A-GTD, and Myo5B-GTD were omitted from the reactions where indicated (lanes 1–4). AU, arbitrary units. C, streptavidin bead–based liposome-tethering assays were employed with Rab11a-His12 and Myo5-GTDs as described in B, but FL-labeled liposomes lacking DOGS-NTA were used instead where indicated (lanes 2 and 4). D–G, fluorescence microscopy was performed as described in Fig. 7, F–K, with Rh-labeled liposomes (0.4 mm lipids), FL-labeled liposomes (0.4 mm lipids), Rab11a-His12 (3 μm), and Myo5A-GTD (D and E) or Myo5B-GTD (F and G) (each at 3 μm). Rh-labeled liposomes lacking DOGS-NTA were used instead where indicated (E and G). Scale bars: 10 μm.