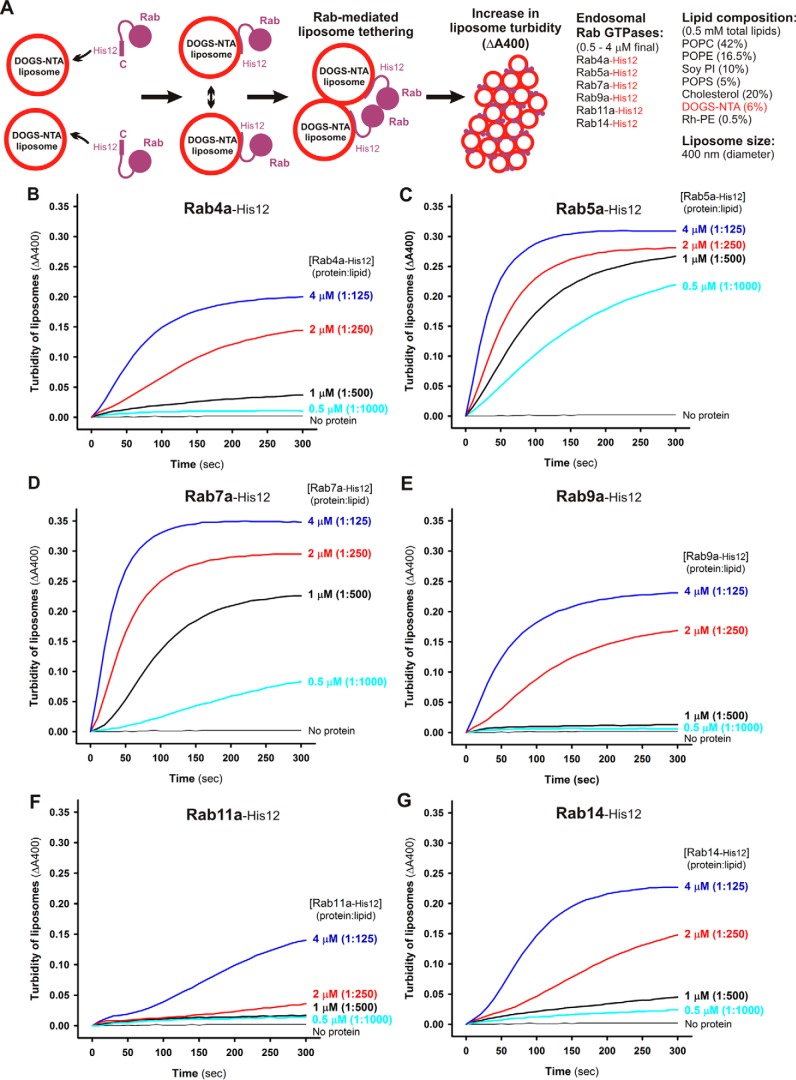
Figure 2.
Endosomal Rab GTPases directly initiate membrane tethering by themselves in a chemically defined reconstitution system. A, schematic representation of liposome turbidity assays for testing Rab-mediated liposome tethering (shown in B–G). B–G, endosomal Rab-His12 proteins (each at 0.5–4 μm), Rab4a-His12 (B), Rab5a-His12 (C), Rab7a-His12 (D), Rab9a-His12 (E), Rab11a-His12 (F), and Rab14-His12 (G), were incubated with synthetic liposomes bearing physiological mimic lipid composition (400 nm diameter, 0.5 mm lipids) in RB150 containing MgCl2 (5 mm) and DTT (1 mm) at room temperature for 300 s. During incubation, turbidity changes in the Rab-liposome–mixed reactions were monitored by measuring the absorbance at 400 nm. The protein-to-lipid molar ratios used for these turbidity reactions were from 1:1000 to 1:125 as indicated.