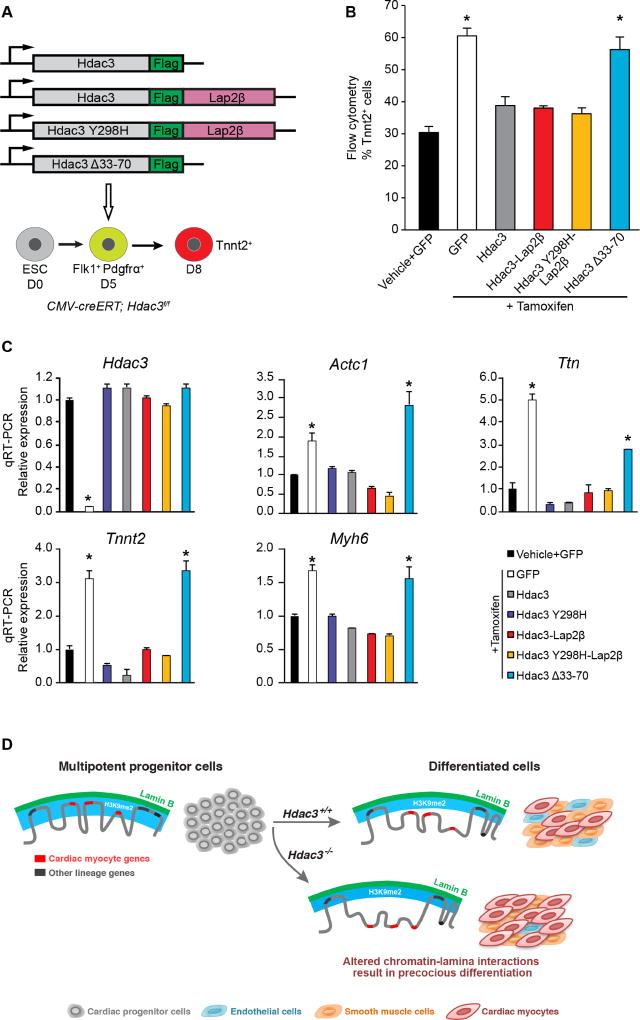
Figure 7. Hdac3 requires interaction with the nuclear lamina to repress cardiac differentiation.
(A) Schema of Hdac3 constructs transduced at day 5 of differentiation with simultaneous tamoxifen-mediated Hdac3 deletion. (B) Percentage of Tnnt2+ cardiac myocyte cells on day 8 of differentiation measured by flow cytometry in cells with vehicle or tamoxifen and indicated constructs; all Hdac3 constructs rescue Hdac3 deletion precocious cardiogenesis phenotype, except Hdac3Δ33-70. (C) Gene expression analysis (qRT-PCR) of CM genes and Hdac3 from day 8 cells with vehicle or tamoxifen and indicated constructs; rescue of cardiac gene expression by all constructs, except Hdac3Δ33-70. Data represent mean ± SEM, n≥3 replicates in (B), (C). * p< 0.05, all statistical comparisons are to vehicle+GFP control, analyzed by one-way ANOVA with a Tukey post-hoc test. (D) Differentiation of multipotent CPCs depends on Hdac3 to orchestrate simultaneous release of genomic regions containing lineage-specific loci from the nuclear lamina. Hdac3 tethers chromatin to the lamina. Without Hdac3, cardiac-specific genes lose contact with the nuclear lamina, are less frequently found within the H3K9me2-marked layer of peripheral heterochromatin, and are more likely to be transcriptionally active.