Fig. 2.
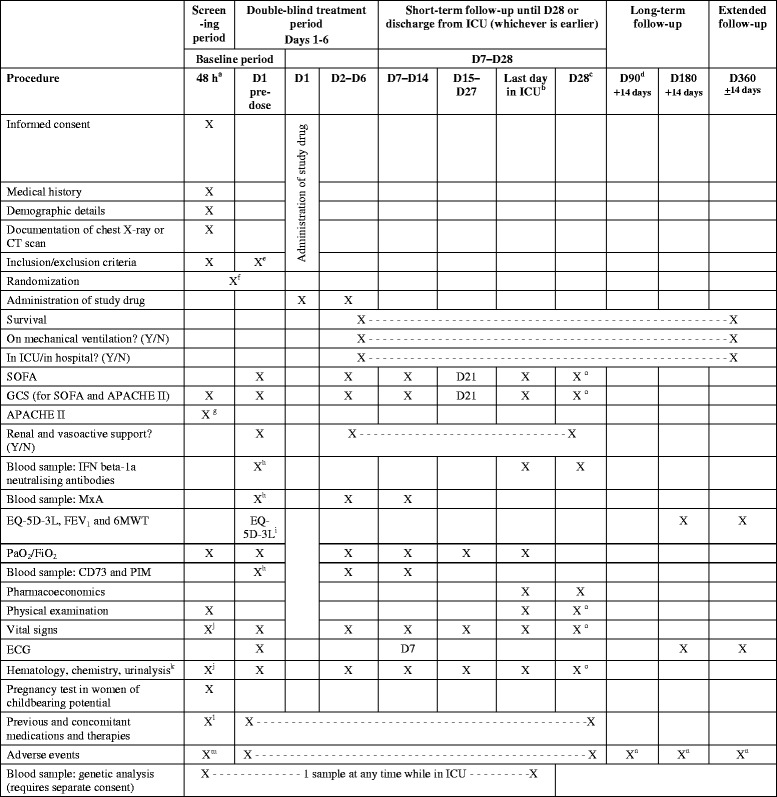
Schedule of procedures. aNo more than 48 hours may elapse between confirmation of moderate or severe ARDS and administration of the first dose of study drug. bThese assessments will be done on the day the patient leaves the ICU, which will either be on D28 or earlier, according to the clinical progress of the patient. If the patient is still in the ICU on D28, the next visit or telephone contact will be at D90. If a patient leaves the ICU before D28, the survival status and other endpoints must be assessed on D28. cD28 procedures apply for patients leaving the ICU before D28 and for patients withdrawing from the study before D28. For patients withdrawing from the study before D28 a sample should be taken for neutralizing antibodies on the day they leave the ICU. dD90 can either be a visit or telephone contact. eReconfirm inclusion/exclusion criteria before dosing, including that patient requires mechanical ventilation and is in the ICU. fRandomize after consent obtained and once eligibility criteria confirmed. gWithin 24 hours of ICU admission. h1 hour pre-dose. iBaseline EQ-5D-3L to be obtained from relatives and checked later with patient. jFor APACHE II scoring. kSamples should be taken in the morning between 04:00 and 10:00. lMedicines and therapies in previous month. mAdverse events will be recorded after informed consent is obtained. nDeaths are reported as SAE. 0if it is possible to be performed by the investigator. APACHE II Acute Physiology and Chronic Health Evaluation, CD Cluster of differentiation, CT Computerized tomography, D Study day, ECG Electrocardiogram, EQ-5D-3L EuroQol 5-Dimensions 3-Levels questionnaire, FEV1 Forced expiratory volume in 1 second, GCS Glasgow Coma Scale, ICU Intensive care unit, IFN Interferon, MxA Myxovirus resistance protein A, PaO2/FiO2 Partial pressure of oxygen/fraction of inspired oxygen, PIM Potential inflammatory marker, SOFA Sequential Organ Failure Assessment, 6MWT 6-minute walk test
