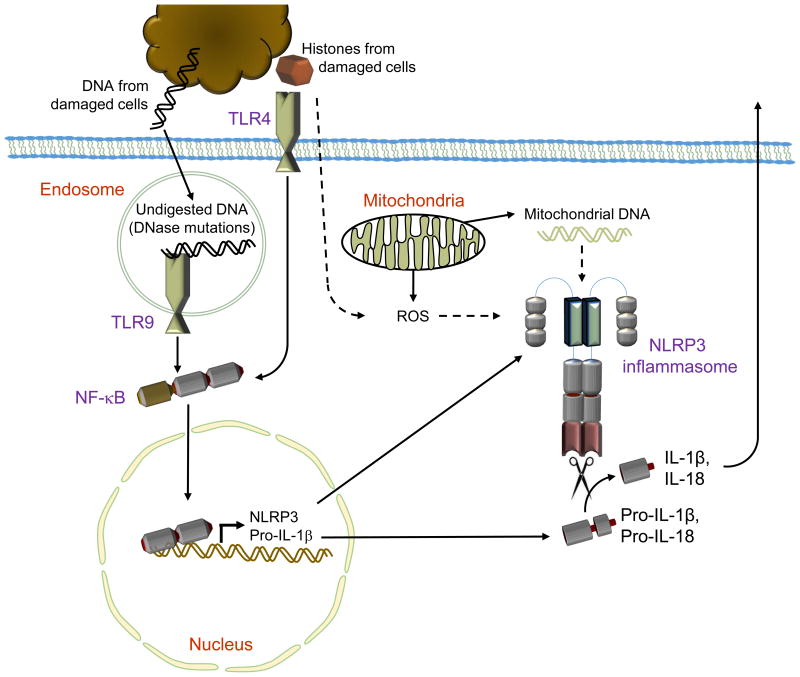
Figure 2. NLRP3 activation by mitochondrial DNA and histones.
NLRP3 inflammasome activation requires two signals in the form of priming the expression of NLRP3 and pro-IL-1β as well as a second damage signal for NLRP3 activation. During sterile inflammation, DNA and histones derived from damaged host cells can prime the NLRP3 inflammasome through TLR-9 or TLR4-mediated increases in NLRP3 and pro-IL-1β expression. The NLRP3 inflammasome can be activated by the presence of cytoplasmic nucleic acids. During sterile inflammation, mitochondrial damage releases mitochondrial DNA (mtDNA) into the cytoplasm where it activates NLRP3. The mechanism of mtDNA-mediated NLRP3 activation is not clear but likely hinges on unknown adaptor proteins or the common signals of potassium efflux or reactive oxygen species generation. NLRP3 activation can also result from histones' ability to damage the cell membrane, but the exact mechanisms are unknown.