Fig. 5.
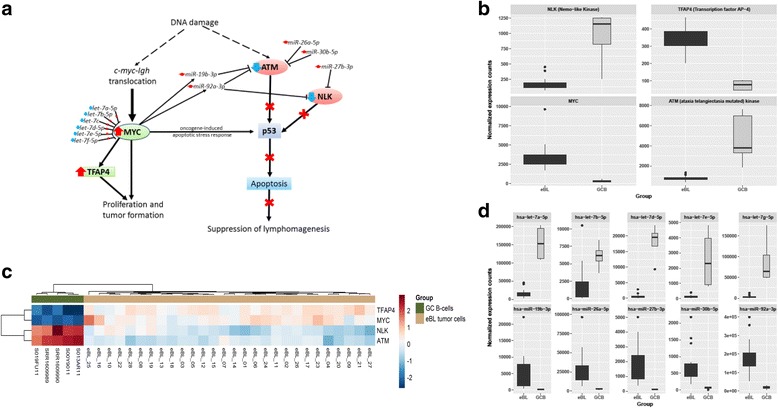
Aberrant transcriptome expression pivotal to eBL lymphomagenesis. a Schematic illustration of the aberrant gene expression and miRNA mediated regulatory changes that would initiate lymphomagenesis as a result of DNA damage. Combined loss of p53 function due to small interfering RNA-mediated regulation of ATM and NLK together with upregulation of TFAP4, would facilitate survival of cells with the c-myc-Igh chromosomal translocation and MYC induced cell cycle progression initiating eBL tumor development. ATM checkpoint kinase, transduces genomic stress signals to halt cell cycle progression in response to DNA damage. It is critical in the regulation of apoptosis and lymphomagenesis in c-myc induced lymphomas. ATM is downregulated in eBL and it is targeted by 4 miRs that are Upregulated in eBL. NLK is required for the upregulation of P53 expression in response to DNA damage. It interacts with P53 to enhance its stability and activity by abrogating MDM2 mediated degradation. NLK is downregulated in eBL tumor cells and also targeted by 2 miRs that are upregulated in eBL tumor cells. TFAP4/AP4 is a central mediator of cell cycle progression in response to c-MYC activation. b RNA seq. Expression counts of MYC, TFAP4, ATM and NLK in eBL tumor cells and GC B cells. c Hierarchical clustering of eBL and GC B cells based on the expression profiles of MYC, TFAP4, ATM and NLK also revealed a clear separation of the two groups. d. miRNA seq. Expression counts of hsa-miR-26a-5p, hsa-miR-27b-3p, hsa-miR-30b-5p, miR-17~92-cluster members (hsa-miR-19b-3p, and hsa-miR-92a-3p), and let-7-family miRs (hsa-let-7a-5p, hsa-let-7b-5p, hsa-let-7d-5p, hsa-let-7e-5p, and hsa-let-7 g-5p) in eBL tumor cells and GC B cells
