Abstract
Background
Dipeptidyl peptidase-4 (DPP-4) may be a suitable biomarker to identify people with severe asthma who have greater activation of the interleukin-13 (IL-13) pathway and may therefore benefit from IL-13-targeted treatments. We report the analytical performance of an Investigational Use Only immunoassay and provide data on the biological range of DPP-4 concentrations.
Methods
We assessed assay performance, utilising analyses of precision, linearity and sensitivity; interference from common endogenous assay interferents, and from asthma and anti-diabetic medications, were also assessed. The assay was used to measure the range of serum DPP-4 concentrations in healthy volunteers and subjects with diabetes and severe, uncontrolled asthma.
Results
The total precision of DPP-4 concentration measurement (determined using percentage coefficient of variation) was ≤5% over 20 days. Dilution analysis yielded linear results from 30 to 1305 ng/mL; the limit of quantitation was 19.2 ng/mL. No notable endogenous or drug interferences were observed at the expected therapeutic concentration. Median DPP-4 concentrations in healthy volunteers and subjects with asthma or Type 1 diabetes were assessed, with concentrations remaining similar in subjects with diabetes and asthma across different demographics.
Conclusion
These analyses indicate that the ARCHITECT DPP-4 Immunoassay is a reliable and robust method for measuring serum DPP-4 concentration.
Abbreviations: BGG, bovine gamma globulin; BMI, body mass index; CI, confidence interval; CLSI, Clinical Laboratory Standards Institute; CV, coefficient of variation; DPP-4, dipeptidyl peptidase-4; HAMA, human anti-mouse antibodies; Ig, immunoglobulin; IL-13, interleukin-13; IUO, Investigational Use Only; LoB, Limit of Blank; LoD, Limit of Detection; LoQ, Limit of Quantitation; mAb, monoclonal antibody; PI, prediction interval; RF, rheumatoid factor; RLU, relative light units; SRT, serum tube-red top; SST, serum separator tube; Th2, T-helper-2
Keywords: Asthma, Automated immunoassay, Biomarker, Dipeptidyl peptidase-4, IL-13
Graphical abstract
Highlights
-
•
DPP-4 may be a suitable biomarker for identifying people with IL-13-driven asthma.
-
•
The new automated DPP-4 immunoassay is performed on the ARCHITECT i System.
-
•
This Investigational Use Only assay reliably measures serum DPP-4 concentrations.
-
•
This assay could be used to identify subjects who might respond to tralokinumab.
-
•
This assay is being utilised in Phase III severe asthma studies of tralokinumab.
1. Introduction
Interleukin-13 (IL-13) is a cytokine, secreted in large quantities by CD4+ T-helper-2 (Th2) cells in patients with Th2-driven asthma or eosinophilic inflammation [1]. Increased IL-13 mRNA expression and protein concentration in bronchial biopsies, sputum and bronchoalveolar lavage fluid from patients with asthma, compared with healthy individuals, supports a role for IL-13 in the pathophysiology of some types of asthma [2], [3], [4], [5]. Furthermore, sputum IL-13 concentrations and the number of cells expressing IL-13 in the bronchial submucosa and airway smooth muscle bundle have been shown to be increased in people with severe asthma [6]. Consequently, identifying people with uncontrolled asthma and increased IL-13 activity might aid in selection of patients who may benefit from anti-IL-13–targeted therapy [7], [8], [9], [10].
During Th2–driven inflammation, IL-13 is expressed locally in inflamed tissue and is present in serum in low concentrations [7], [11]. This creates a challenge for its use as a biomarker of asthma, particularly as serum IL-13 concentrations remain similar between healthy volunteers and people with asthma [11]. An alternative, readily measureable biomarker is therefore required in order to identify those people with IL-13–driven asthma. Dipeptidyl peptidase-4 (DPP-4) production by airway cells is induced, in vitro, by IL-13 [8]. DPP4 gene expression has been shown to be upregulated in the nose and bronchi of children with asthma and in the bronchi of adults with asthma, which also correlated with IL-13 mRNA upregulation [12], [13]. Therefore, DPP-4 may prove to be a suitable biomarker for identifying people with IL-13-driven asthma who could benefit from IL-13-targeted treatments. Indeed, the relationship between serum DPP-4 concentrations and response to an anti–IL-13–targeted treatment has previously been shown in a Phase IIb study of tralokinumab, an anti–IL-13 monoclonal antibody (mAb) in subjects with severe, uncontrolled asthma [14].
DPP-4 (also known as adenosine deaminase complexing protein 2 or CD26) is a 766-amino acid membrane serine peptidase, highly expressed in the lung, kidney, liver and small intestines [15]. It is an integral type II glycoprotein homodimer anchored to the cell membrane by its signal peptide [15]. DPP-4 can be shed from the cell membrane into circulation in a soluble, active form [15], facilitating its measurement as a soluble biomarker. DPP-4 regulates glucose metabolism through degradation of incretin peptides [16], [17] and may also have enzymatic functions in immune system modulation, cardiovascular physiology and tumour biology [18], [19], [20], [21].
We describe the development of the ARCHITECT DPP-4 Investigational Use Only (IUO) Immunoassay, currently in use to assess the utility of DPP-4 as a biomarker in Phase III studies investigating tralokinumab in subjects with severe uncontrolled asthma (NCT02161757, NCT02194699 [10]). We report the analytical performance of the assay and provide data on the biological variability of serum DPP-4 concentrations across different subject demographics.
2. Materials and methods
2.1. Assay description
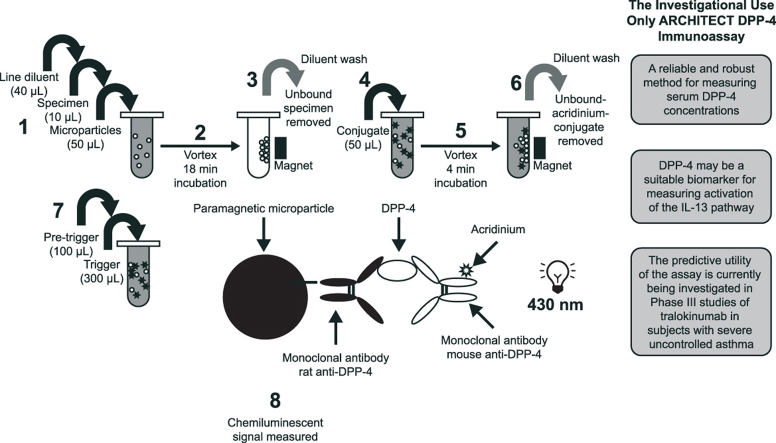
The IUO ARCHITECT DPP-4 Immunoassay was developed for use with the ARCHITECT Immunoassay i System (Abbott Laboratories, Abbott Park, IL) [22]. The assay determines serum DPP-4 concentration using a two-step dual non-competing mAb sandwich process with methodology that has previously been described [23]. Briefly, assay samples and standards were diluted 10-fold with line diluent and microparticles; DPP-4 was captured by rat anti–DPP-4 mAb-coated paramagnetic microparticles and detected with acridinium-labelled mouse anti–DPP-4 mAb. A chemiluminescent signal, reportable as relative light units, directly correlates with the amount of DPP-4 present (Fig. 1). The mAbs used in the immunoassay were generated by MedImmune (Gaithersburg, MD) using a hybridoma platform and purified using affinity chromatography with Protein G and Protein A for the rat and mouse mAb, respectively [24].
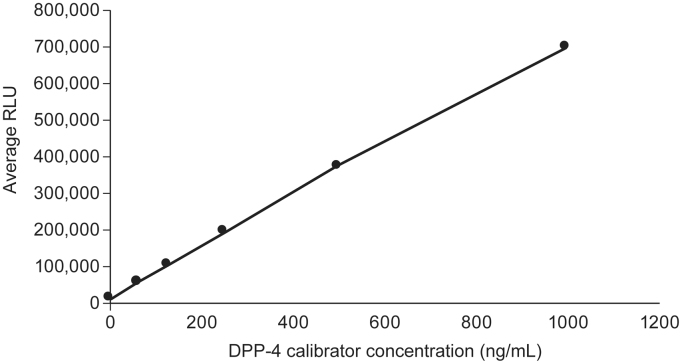
Fig. 1.
Relationship between relative light units (RLU) and dipeptidyl peptidase-4 (DPP-4) concentrations.
The assay was standardised using a commercially available purified recombinant human dimeric DPP-4 protein (NCBI accession number: CAA43118), with a C-terminal His-tag for purification (Bio-Techne Inc., MN, USA), produced from a mouse myeloma-derived NS0 cell line. The assay had a calibration range of 0–1000 ng/mL, selected to reflect the baseline concentrations of DPP-4, prior to treatment with agents, of the population for which the assay is intended. The assay utilised subjects in a Phase IIb study of tralokinumab who had DPP-4 concentrations (measured using the IUO ARCHITECT DPP-4 Immunoassay) ranging from 109 ng/mL to 580 ng/mL (NCT01402986 [14]). The assay is fully automated with a throughput of 200 tests per hour [22].
2.2. Assay performance
2.2.1. Samples
Samples from healthy volunteers were obtained from ProMedDX, LLC (Norton, MA). Samples from subjects with asthma and Type 1 diabetes, used in DPP-4 concentration assessments, were obtained from a Phase IIb study of tralokinumab in subjects with severe uncontrolled asthma receiving concomitant high-dose fluticasone and salmeterol (NCT01402986 [14]) and from ProMedDX, LLC, respectively. Additional samples, collected for a preanalytical in-house study to confirm specimen-handling procedures, were provided by consenting volunteers with self-reported asthma at Abbott Laboratories (Abbott Park, IL). All samples evaluated in this assay were serum from standard or serum separator tubes which were collected in line with approved protocols from institutional review boards. No other specimen matrices or tubes were evaluated.
2.2.2. Precision testing
Assay precision was evaluated according to Clinical Laboratory Standards Institute (CLSI) guideline EP05-A2 [25] using methods previously described [23]. Tests were performed with the aim of achieving a coefficient of variation (CV) of ≤10% for total precision. Low and high DPP-4 concentration controls were prepared by spiking recombinant antigen into the artificial matrix of phosphate buffered saline (pH 7.2), 1% bovine serum albumin (BSA), and azide preservative. The zero calibrator is artificial matrix without the recombinant DPP-4. Controls had target values of 125 ng/mL and 500 ng/mL, respectively. A native human serum sample panel was also included. Analyses were carried out over 20 days, conducting two runs per day and three repetitions per control or panel level within each run.
2.2.3. Dilution linearity
Dilution linearity of three different native endogenous serum samples was assessed according to CLSI guideline EP06-A [26]. Samples of human serum were spiked with recombinant DPP-4 and then diluted with wash buffer to concentrations between approximately 0 ng/mL and 1300 ng/mL. Tests were performed with the aim of achieving a difference of ≤10% between expected and observed values.
2.2.4. Spiked recovery
Ten serum specimens from healthy volunteers were spiked with recombinant DPP-4 up to a concentration of 500 ng/mL. The percentage recovery of spiked samples was assessed [27] with the aim of achieving spiked recovery of between 80% and 120%.
2.2.5. Interferents
Interference tests were designed with the aim of achieving a mean percentage difference in DPP-4 concentration of ≤10%, in the presence and absence of interferent.
2.2.5.1. Common interfering substances for clinical specimens
Potential interference from haemoglobin (>500 mg/dL), protein (>12 g/dL), bilirubin (>20 mg/dL) and triglyceride (>3000 mg/dL) was assessed using haemolysate, bovine gamma globulin (BGG), conjugated and unconjugated bilirubin, and intralipid (Baxter, USA), respectively. Intralipid is a triglyceride solution consisting of 20% intravenous fat emulsion and was used as a spiking solution to prepare triglyceride test samples. The concentration of interferents in each sample was confirmed by Abbott Clinical platforms. Tests were performed according to CLSI EP07-A2 guidelines [28]; the methodology for this analysis has previously been described [23].
2.2.5.2. Drug interferents
Cross-reactivity with selected common asthma medications was assessed at therapeutically relevant concentrations. These included salbutamol (drug tested at concentration of 1.67 µmol/L), fluticasone propionate (345 pg/mL), prednisone (0.82 μmol/L), desloratadine (0.97 μmol/L), montelukast (0.90 μg/mL), ipratropium bromide (300 pg/mL), cromolyn sodium (0.03 μg/mL), theophylline (222 μmol/L) and omalizumab (0.3 mg/mL). Concentrations were based on CLSI guideline EP07-A2 [28] or, alternatively, were at least three times the peak concentration following therapeutic dosage [23]. Four anti-diabetic medications were also assessed for interference (sitagliptin, saxagliptin, vildagliptin and teneligliptin) at concentrations up to three times those observed for the highest reported therapeutic dosage.
2.2.5.3. Interference from antibodies
Human anti-mouse antibodies (HAMA; n = 20) and rheumatoid factor (RF) antibodies (n = 20) were compared with current (0.5 mg/mL) and twice the amount of rat immunoglobulin (Ig) G blocking agent (1.0 mg/mL). A two-sided 95% confidence interval (CI) of ≤10% around the mean percentage difference in samples from blocking agent would indicate that interference from the tested antibodies was not significant.
2.2.5.4. Interference from proteins related to DPP-4
Seprase, a protein with homology to DPP-4 ≥50%, was analysed for cross-reactivity in the immunoassay. An endogenous serum sample was spiked to 1000 ng/mL with seprase and compared with the same sample, containing an equal amount of 0.9% saline. A two-sided 95% CI of ≤10% around the percentage difference of the two samples was calculated.
2.2.6. Limit of blank (LOB), limit of detection (LOD) and limit of quantitation (LOQ)
LOB was analysed using goat serum and zero calibrator matrix. Both matrices are known to be true zero samples and were used to fully assess the variability in concentration of these zero-analyte matrices in the ARCHITECT DPP-4 assay [29]. DPP-4 aggregate was spiked into goat serum to create a set of panels of DPP-4 concentrations at 19.2, 20.2, 31.8, 33.5, 49.0, 53.7, 60.2, 63.6, 68.9 and 73.1 ng/mL. Samples were tested in replicates of 10 each per run on each of two different instrument lots with two reagent lots over 6 days. LOD and LOQ were evaluated according to CLSI guideline EP17-A2 [29] using methods previously described [23].
2.3. Characterisation of assay antibodies
Full-length predominantly dimeric human DPP-4, purchased from R&D Systems (Minneapolis, MN), was purified through size exclusion chromatography using a Superdex 200 column on an Akta Purifier 10 instrument. As this was a preparative separation and the oligomeric forms were not baseline separated, each fractionated sample was not pure monomer, dimer and oligomer. The samples were then electrophoresed in reduced and non-reduced forms. Western blots were probed with assay capture and detection mAbs and developed as previously described [23] to detect monomeric (approximately 105 kDa), dimeric (approximately 210 kDa) and multimeric (>315 kDa) forms of DPP-4. High-performance liquid chromatography (HPLC) size-fractionation of specimen samples for subsequent activity measurements was accomplished through a Tosoh G3000 SWxL column with standard phosphate-buffered saline as the mobile phase.
2.4. Specimen handling
Serum samples from volunteers with self-reported asthma (n = 21) were used to assess sample stability at room (30 °C) and refrigeration (2–8 °C) temperatures, and following repeated freeze/thaw cycles. Specimens frozen at –70 °C for at least 12 h were transferred to room temperature for a minimum of 1 h, prior to testing. It was necessary for upper and lower 95% CIs around the mean percentage difference in DPP-4 concentration to be ≤10% to support storage at a given temperature.
2.4.1. Sample stability at room and refrigeration temperatures
Samples were stored using normal serum tube-red top (SRT) and serum separator tube (SST) types [23]. Samples were analysed “on” and “off” the clot to mimic “worst-case” sample handling techniques and potential variation in handling, respectively. Sample stability was assessed by calculating the percentage change in DPP-4 concentration over 7 days; samples with a two-sided CI of >10% from baseline were considered unstable. Samples were stored at room temperature for 8 h prior to the storage temperature analysis [23].
2.4.2. Freeze/thaw cycle stability
Samples were frozen and evaluated “off” the clot [23]. Four freeze/thaw cycles were conducted for serum specimens frozen at −10 °C and −70 °C [23]. Mean percentage differences were calculated after each cycle.
2.5. DPP-4 concentration assessments in healthy volunteers and subjects with severe, uncontrolled asthma, or Type 1 diabetes
Serum samples from apparently healthy volunteers (n = 757) and subjects with severe, uncontrolled asthma treated with inhaled corticosteroids (n = 447) were evaluated using the ARCHITECT DPP-4 Immunoassay and analysed by sex, age and ethnicity (healthy volunteers only), providing mean, median and range of serum DPP-4 concentrations for each population. DPP-4 is a therapeutic target for a class of anti-diabetic drugs that inhibit DPP-4; therefore, DPP-4 was measured to survey the analyte concentration in a diabetic population (n = 100). Subjects were evaluated by sex, age and body mass index (BMI).
3. Results
3.1. Assay performance
3.1.1. Precision testing
Total precision (measured using percentage CV) was ≤5% over 20 days for the low and high DPP-4 controls and native human serum sample panel analysed (Table 1).
Table 1.
20-day assay precision evaluation summary.
| Lot | N | Mean DPP-4 concentration (ng/mL) | %CV (SD) |
|||
|---|---|---|---|---|---|---|
| Within run | Between run | Between day | Totala | |||
| Low concentration control (Target: 125 ng/mL) | ||||||
| 1 | 240 | 121.7 | 3.7 (4.5) | 0 (0) | 1.7 (2.0) | 4.1 (4.9) |
| 2 | 240 | 119.6 | 4.2 (5.1) | 1.1 (1.3) | 1.9 (2.2) | 4.8 (5.7) |
| 3 | 240 | 120.0 | 3.5 (4.1) | 1.6 (2.0) | 1.8 (2.1) | 4.2 (5.1) |
| High concentration control (Target: 500 ng/mL) | ||||||
| 1 | 240 | 477.5 | 3.3 (15.9) | 1.5 (7.1) | 1.6 (7.6) | 4.0 (19.0) |
| 2 | 240 | 488.7 | 3.5 (16.9) | 0 (0) | 1.4 (7.0) | 3.7 (18.3) |
| 3 | 240 | 489.1 | 3.5 (17.0) | 0.9 (4.3) | 1.8 (8.9) | 4.0 (19.7) |
| Human serum panel | ||||||
| 1 | 240 | 285.1 | 3.5 (9.9) | 1.2 (3.4) | 1.6 (4.7) | 4.0 (11.5) |
| 2 | 240 | 296.7 | 3.4 (10.1) | 1.4 (4.1) | 1.2 (3.7) | 3.9 (11.5) |
| 3 | 240 | 289.8 | 3.3 (9.6) | 1.0 (3.0) | 1.7 (4.8) | 3.9 (11.2) |
CV: coefficient of variation; DPP-4: dipeptidyl peptidase-4; SD: standard deviation.
Total %CV (SD) contains within run, between run and between day variance components.
3.1.2. Dilution linearity
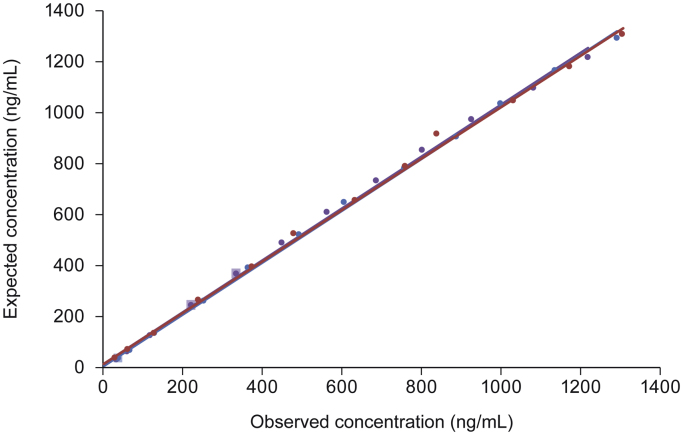
Dilution analysis of three serum samples spiked with recombinant DPP-4 gave linear results down to a concentration of approximately 30 ng/mL. Nearly all samples analysed had dilution-adjusted observed concentrations at each dilution level that varied by <10% compared with the expected value (Fig. 2).
Fig. 2.
Relationship between expected and least-squares mean observed dipeptidyl peptidase-4 (DPP-4) concentrations following serial dilution of samples A ( ), B (
), B ( ) and C (
) and C ( ). The dilution factor was used to determine the expected results of the undiluted sample at the dilutions made and compared with the observed or assayed result. Lighter squares represent samples in which observed values differ from expected values by ≥10%.
). The dilution factor was used to determine the expected results of the undiluted sample at the dilutions made and compared with the observed or assayed result. Lighter squares represent samples in which observed values differ from expected values by ≥10%.
3.1.3. Spiked recovery
The mean (range) recovery of DPP-4 spiked into endogenous serum was 92% (64–107%); nine out of 10 samples analysed were within the targeted DPP-4 recovery range of 80–120%.
3.1.4. Interferents
3.1.4.1. Common interfering substances for clinical specimens and drug interferents
All endogenous interferents and common asthma medications evaluated were not considered to interfere with the assay, demonstrating a mean percentage interference of <10% (Table 2). Furthermore, three of the anti-diabetic medications (vildagliptin, saxagliptin and teneligliptin) analysed were also not considered to be interferents at concentrations three times greater than those resulting from the highest recommended therapeutic dosage. Sitagliptin demonstrated interference of <5% at its greatest reported therapeutic dosage; this increased to >50% interference at concentrations observed with triple the maximum reported therapeutic dosage (Table 2).
Table 2.
Mean percentage difference in DPP-4 concentration with and without common endogenous interferents, asthma medications and anti-diabetic medications.
| Interferent | Interferent concentration | Mean % difference in DPP-4 concentration |
|---|---|---|
| Endogenous interferents | ||
| Unconjugated bilirubin | >20 mg/dL | –0.6 |
| Conjugated bilirubin | >20 mg/dL | 4.0 |
| Haemoglobin | >500 mg/dL | –8.0 |
| Total protein | >12,000 mg/dL | 1.0 |
| Triglycerides | >3000 mg/dL | –3.0 |
| Asthma medications | ||
| Salbutamol | 1.67 µmol/L | –0.8 |
| Fluticasone | 345 pg/mL | –0.7 |
| Prednisone | 0.82 µmol/L | –1.8 |
| Desloratidine | 0.97 µmol/L | –3.6 |
| Montelukast | 0.90 µg/mL | 1.7 |
| Ipratropium bromide | 300 pg/mL | –0.2 |
| Cromolyn sodium | 0.03 µg/mL | –0.1 |
| Theophylline | 222 µmol/L | 0.9 |
| Omalizumab | 0.3 mg/mL | –0.1 |
| Anti-diabetic medications | ||
| Vildagliptin (3 X RTD) | 1191 ng/mL | –1.9 |
| Saxagliptin (3 X RTD) | 141 ng/mL | 3.83 |
| Teneligliptin (3 X RTD) | 825 ng/mL | 4.01 |
| Sitagliptin (3 X RTD) | 1439 ng/mL | –56.0 |
| Sitagliptin (2 x RTD) | 720 ng/mL | –30.1 |
| Sitagliptin (1 x RTD) | 480 ng/mL | –4.0 |
DPP-4: dipeptidyl peptidase-4; RTD: recommended therapeutic dosage.
3.1.4.2. Interference from antibodies
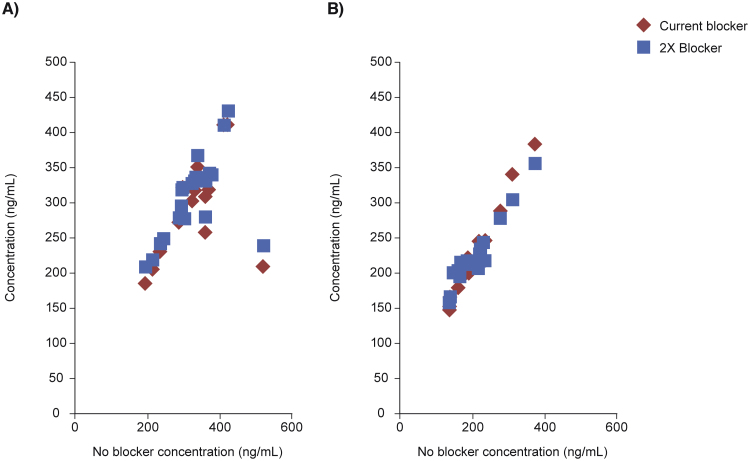
No substantial interference from HAMA or RF samples was seen (Fig. 3). The two-sided 95% CI for the percentage difference in HAMA and RF samples from blocking agent was 3.0, 8.0 and –1.3, 8.6, respectively.
Fig. 3.
Regression analysis of potential interference from human anti-mouse antibodies (HAMA) (A) and rheumatoid factor (RF) (B). 0.5 mg/mL rat immunoglobulin G (IgG) was used as a blocker.
3.1.4.3. Interference from seprase
At a concentration of 1000 ng/mL, seprase was not considered to interfere with the assay; mean percentage interference was ≤10% (95% CI in presence of analyte: –1.7, 1.9; 95% CI in absence of analyte: –0.05, 0.03).
3.1.5. LOB, LOD and LOQ
The mean LOB was 0.4 ng/mL (range: 0.2–0.6 ng/mL) and mean LOD was 2.0 ng/mL (range: 1.8–2.3 ng/mL). The LOQ for this assay, calculated using 10 serum DPP-4 panel concentrations, was 19.2 ng/mL.
3.2. Characterisation of DPP-4 recombinant protein and epitope mapping
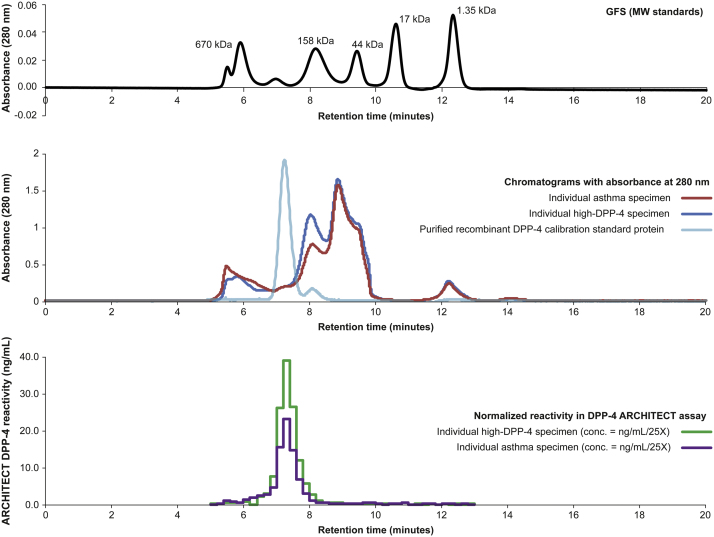
Before sizing-purification, the recombinant DPP-4 protein standard used in this analysis consisted of monomer, dimer and high molecular weight aggregate complexes with approximate distributions of 10%, 85% and 5%, respectively. The high molecular weight aggregate was less reactive in the immunoassay (59.9 ng/mL) compared with the dimeric (699.9 ng/mL) and monomeric forms (403.2 ng/mL). The individual DPP-4 high and asthma specimen samples, analysed for reactivity in the assay, align with the purified recombinant dimeric DPP-4 standard protein in the sizing-HPLC chromatogram at 280 nm (Fig. 4).
Fig. 4.
Absorbance (280 nm) and normalised immunoreactivity of individual specimens from subjects with asthma and concentrations of dipeptidyl peptidase-4 (DPP-4) above the baseline median. Abbreviation: GFS: gel filtration standard; MW: molecular weight.
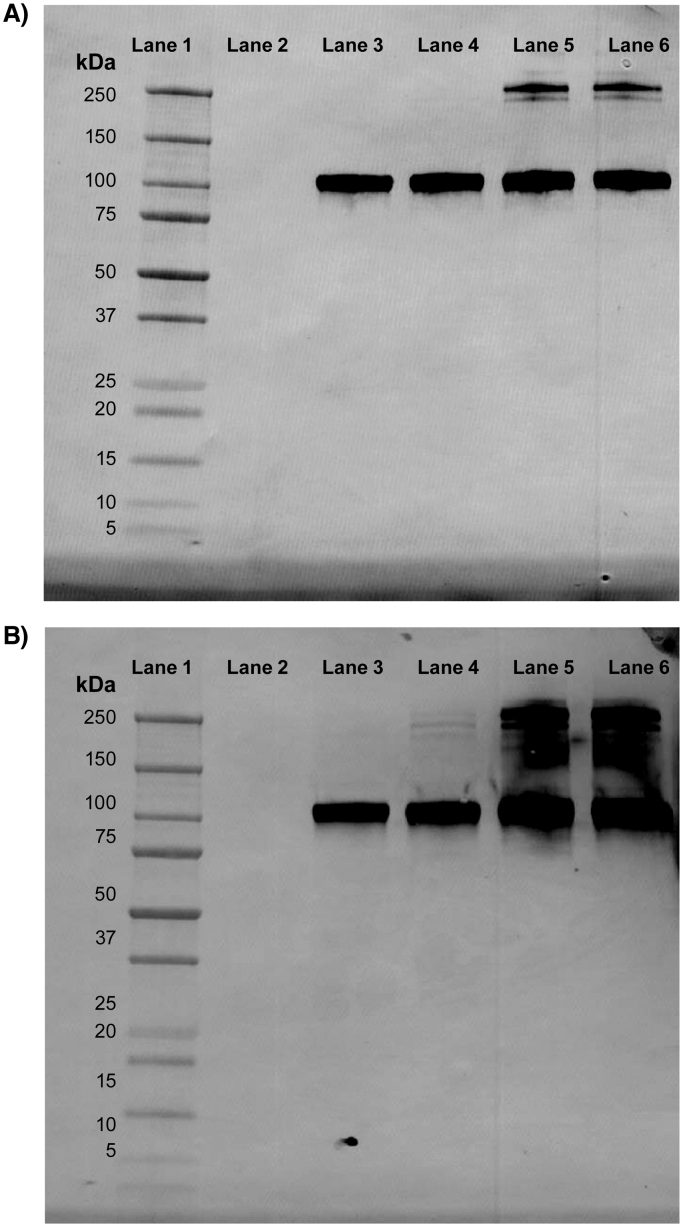
Western blot analyses showed that antibodies used in the DPP-4 assay detect both monomeric and dimeric forms in the reduced and non-reduced state (Fig. 5). The regions of the binding sites of DPP-4 to assay antibodies have also been identified using epitope mapping; the two mAbs have distinct epitopes (data not shown).
Fig. 5.
Western blot analyses showing detection of monomeric (approximately 105 kDa) and dimeric dipeptidyl peptidase-4 (DPP-4) forms (approximately 210 kDa) by detection (A) and capture (B) antibodies, respectively, used in the ARCHITECT DPP-4 Immunoassay. Lane 1: Dual Xtra Molecular Weight Markers; Lane 2: Empty; Lane 3: DPP-4 fragment (reduced and boiled); Lane 4: DPP-4 fragment (reduced and non-boiled); Lane 5: DPP-4 fragment (non-reduced and boiled); Lane 6: DPP-4 fragment (non-reduced and non-boiled). Abbreviation: DPP-4: dipeptidyl peptidase-4.
3.3. Specimen handling
3.3.1. Sample stability at room and refrigeration temperatures
Mean percentage difference from baseline in DPP-4 concentrations increased with time but remained ≤0.1% for samples kept “on” and “off” the clot at room and refrigeration temperature (Table 3). Only the “off the clot” SST samples, stored at room and refrigeration temperatures, were unstable by Day 7 (Table 3).
Table 3.
Differences in DPP-4 concentration from baseline at room and refrigeration temperatures, and after passing through four freeze/thaw cycles (N = 21).
| Day | SRT |
SST |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 7 | 1 | 2 | 3 | 4 | 7 | |||||||
| Room temperature (30 °C) | ||||||||||||||||
| “On” the clot | ||||||||||||||||
| Mean | 0.01 | 0.05 | 0.06 | 0.05 | 0.08 | 0.01 | 0.03 | 0.04 | 0.04 | 0.09 | ||||||
| 2-sided 95% CIa | –0.5, 3.3 | 2.6, 6.4 | 5.0, 7.7 | 3.8, 7.0 | 5.4, 9.9 | –0.7, 3.0 | 0.6, 4.9 | 2.7, 6.0 | 2.4, 6.1 | 7.3, 10.8 | ||||||
| “Off” the clot | ||||||||||||||||
| Mean | 0.01 | 0.03 | 0.04 | 0.03 | 0.08 | 0.02 | 0.05 | 0.04 | 0.05 | 0.10 | ||||||
| 2-sided 95% CIa | –0.2, 3.0 | 0.9, 5.2 | 2.0, 6.7 | 0.6, 4.9 | 6.1, 9.6 | 0.2, 3.9 | 2.7, 7.4 | 2.9, 5.7 | 3.3, 7.0 | 8.1, 11.4 | ||||||
| Refrigeration temperature (2–8 °C) | ||||||||||||||||
| “On” the clot | ||||||||||||||||
| Mean | 0.01 | 0.02 | 0.04 | 0.04 | 0.09 | 0.01 | 0.01 | 0.03 | 0.04 | 0.09 | ||||||
| 2-sided 95% CIa | –0.1, 2.7 | 0.1, 3.5 | 2.3, 5.2 | 1.6, 6.2 | 7.7, 11.0 | –1.2, 2.6 | –1.3, 3.7 | 0.5, 4.8 | 1.1, 6.1 | 5.9, 11.6 | ||||||
| “Off” the clot | ||||||||||||||||
| Mean | 0.00 | 0.00 | 0.01 | 0.00 | 0.06 | 0.02 | 0.02 | 0.06 | 0.05 | 0.10 | ||||||
| 2-sided 95% CIa | –1.2, 1.7 | –2.9, 2.2 | –0.9, 3.4 | –2.5, 1.9 | 3.4, 8.2 | 0.8, 3.4 | –0.1, 4.6 | 4.1, 7.9 | 3.3, 7.6 | 8.0, 12.5 | ||||||
| Number of freeze/thaw cycles | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||||||||
| Storage at –10 °C | ||||||||||||||||
| Mean | –0.02 | –0.02 | –0.02 | –0.02 | 0.03 | 0.02 | 0.02 | 0.03 | ||||||||
| 2-sided 95% CIa | –3.0, –0.5 | –3.2, –1.2 | –3.8, –1.0 | –3.0, –0.5 | 1.6, 4.0 | 0.7, 3.6 | 1.3, 3.6 | 1.8, 4.3 | ||||||||
| Storage at –70 °C | ||||||||||||||||
| Mean | –0.03 | –0.03 | –0.02 | –0.03 | 0.02 | 0.03 | 0.02 | 0.03 | ||||||||
| 2-sided 95% CIa | –4.6, –1.6 | –4.3, –1.2 | –3.5, –0.5 | –4.2, –1.4 | 0.4, 3.0 | 1.4, 4.1 | 1.0, 3.5 | 1.1, 4.5 | ||||||||
CI: confidence interval; DPP-4: dipeptidyl peptidase-4; SRT: serum-tube red top; SST: serum separator tube.
Samples with a 2-sided 95% CI of ≤10% were considered stable.
3.3.2. Freeze/thaw cycle stability
Samples stored at −10 °C and −70 °C were stable after passing through four freeze/thaw cycles; 95% CIs remained below the 10% threshold (Table 3).
3.4. DPP-4 concentration assessments in healthy volunteers and subjects with severe, uncontrolled asthma or Type 1 diabetes
The mean DPP-4 concentration in serum samples of apparently healthy volunteers was 343 ng/mL; this value was similar regardless of ethnicity (Table 4A). Concentrations are elevated in subjects aged 16–21 years, compared with the total population (p<0.0001). Males of this age group had the greatest mean DPP-4 concentration (424 ng/mL; Table 4A). The mean DPP-4 concentration was lower in subjects with diabetes, compared with healthy volunteers (319 ng/mL; p < 0.0066; Table 4B), and further decreased in subjects with severe asthma taking inhaled corticosteroids (273 ng/mL; p < 0.0001; Table 4C). However, concentrations remained consistent in subjects with diabetes and asthma across different demographics, with the exception of subjects aged 18–21 years with severe, uncontrolled asthma, where the mean DPP-4 concentration was 345 ng/mL (Table 4C).
Table 4.
Distribution of DPP-4 concentrations in samples from apparently healthy volunteers (A), subjects with Type 1 diabetes (B) and subjects with asthma taking inhaled corticosteroids (C).
|
(A) | ||||
|---|---|---|---|---|
| DPP-4 concentration (ng/mL) |
||||
| Number of samples | Mean (SD) | Median | Range | |
| All | 757 | 343 (91.4) | 328 | 142–747 |
| Sex | ||||
| Female | 384 | 311 (68.6) | 308 | 169–599 |
| Aged 16–21, years | 215 | 329 (61.8) | 319 | 190–517 |
| Male | 373 | 376 (100.1) | 362 | 142–747 |
| Aged 16–21, years | 200 | 424 (99.5) | 421 | 177–747 |
| Age, years | ||||
| 16–21 | 415 | 374 (94.8) | 354 | 177–747 |
| 22–30 | 53 | 323 (78.8) | 316 | 174–599 |
| 31–40 | 91 | 307 (72.6) | 299 | 169–534 |
| 41–50 | 92 | 288 (62.9) | 290 | 189–446 |
| 51–60 | 64 | 315 (73.2) | 307 | 191–571 |
| 61–72 | 42 | 299 (59.8) | 307 | 142–419 |
| Ethnicity | ||||
| African American | 192 | 342 | 320 | 193–708 |
| Asian | 165 | 333 | 327 | 201–582 |
| Caucasian | 200 | 347 | 330 | 142–747 |
| Hispanic | 200 | 348 | 333 | 169–653 |
|
(B) | ||||
|---|---|---|---|---|
| DPP-4 concentration (ng/mL) |
||||
| Number of samples | Mean (SD) | Median | Range | |
| All | 100 | 319 (81.3) | 311 | 71–558 |
| Sex | ||||
| Female | 50 | 323 (78.0) | 310 | 102–558 |
| Male | 50 | 314 (84.9) | 320 | 71–521 |
| Age, years | ||||
| 22–30 | 15 | 302 (77.7) | 302 | 102–409 |
| 31–40 | 15 | 333 (78.7) | 311 | 215–498 |
| 41–50 | 20 | 332 (70.7) | 328 | 217–498 |
| 51–60 | 27 | 326 (80.1) | 310 | 202–558 |
| 61–77 | 23 | 302 (95.9) | 300 | 71–521 |
| BMI (kg/m2) | ||||
| <24.9 | 37 | 328 | 328 | 102–558 |
| 25.0–29.9 | 33 | 323 | 310 | 71–521 |
| >30.0 | 28 | 298 | 302 | 205–449 |
|
(C) | ||||
|---|---|---|---|---|
| DPP-4 concentration (ng/mL) |
||||
| Number of samples | Mean (SD) | Median | Range | |
| All | 447 | 273 (74.3) | 264 | 109–580 |
| Sex | ||||
| Female | 294 | 277 (76.3) | 269 | 109–580 |
| Male | 153 | 265 (69.9) | 254 | 123–495 |
| Age, years | ||||
| 18–21 | 9 | 345 (100.6) | 331 | 207–536 |
| 22–30 | 21 | 273 (78.5) | 261 | 156–509 |
| 31–40 | 76 | 275 (65.7) | 263 | 120–428 |
| 41–50 | 108 | 257 (65.8) | 250 | 109–456 |
| 51–60 | 140 | 281 (81.3) | 274 | 147–580 |
| 61–70 | 75 | 277 (76.6) | 270 | 123–535 |
| 71–80 | 18 | 250 (35.7) | 252 | 184–333 |
| Ethnicity | ||||
| African American | 14 | 273 | 260 | 156–439 |
| American Indian/Eskimo | 27 | 284 | 266 | 177–536 |
| Asian | 156 | 289 | 279 | 171–580 |
| Caucasian | 174 | 249 | 244 | 109–466 |
| Hispanic | 76 | 292 | 286 | 109–535 |
BMI: body mass index; DPP-4: dipeptidyl peptidase-4; SD: standard deviation.
4. Discussion
Using the analyses described in this manuscript, we have demonstrated that the ARCHITECT DPP-4 Immunoassay is a reliable and robust assay for determination of serum DPP-4 concentration. This is indicated by precision levels of approximately 5% CV within the laboratory, and a high assay sensitivity for DPP-4 demonstrated by an LOQ value of 19.2 ng/mL. DPP-4 patient sample stability has been demonstrated to be ≥4 days at room and refrigerated temperatures for the SRT and SST tube types. In addition, DPP-4 patient samples are stable for up to four freeze/thaw cycles. Furthermore, good dilution linearity was observed across the concentration range evaluated.
The common endogenous interferents or asthma medications analysed did not demonstrate substantial interference to the assay (i.e., percentage difference with and without interferent was less than 10%). The anti-diabetic medication sitagliptin showed some assay interference when tested at supratherapeutic concentrations; however, there was no interference at the recommended therapeutic concentration and this concentration is considered to be at the high end of the therapeutic range. The ARCHITECT DPP-4 Immunoassay contains rat IgG blocking agents to prevent interference from HAMA and RF antibodies; our analyses demonstrate that blockage was successful for this assay. Using mAbs from two different species may have contributed to avoiding some types of HAMA interference, as it is unlikely both would be bound by a given HAMA.
The DPP-4 immunoassay offers a two-step approach, reducing the likelihood of high-dosage hook effects [22], minimising the risk of false-negative results in samples with high DPP-4 concentrations [30], reducing non-specific binding and preventing exposure of acridinium to potential interferents [22]. The antibodies used in the assay can detect monomeric, dimeric and high molecular weight aggregate content; however, the majority of the native circulating DPP-4 analysed from subject serum was found in dimeric form (approximately 85%). Epitope mapping analyses have confirmed that the two mAbs used in the assay bind at different distinct sites of the DPP-4 protein and are therefore non-competing.
Some variation was seen in DPP-4 concentration across different subject demographics. Healthy male volunteers aged 16–21 years demonstrated greater DPP-4 concentrations (median DPP-4 concentration: 421 ng/mL) compared with the overall population (328 ng/mL). Furthermore, subjects with Type 1 diabetes and severe, uncontrolled asthma (treated with high doses of fluticasone and salmeterol) demonstrated lower median DPP-4 concentrations of 311 ng/mL and 264 ng/mL, respectively, when compared with healthy volunteers.
To conclude, the analyses within this study indicate that the ARCHITECT DPP-4 assay is a reliable and robust method for measuring serum DPP-4 concentration. The assay is currently being applied to assess the clinical utility of DPP-4 as a predictive biomarker in Phase III studies of tralokinumab in subjects with uncontrolled asthma (NCT02161757, NCT02194699) [10].
Acknowledgments
We thank Lisa Steinbrück (PEPperPRINT) for her support in the epitope mapping analyses, and Susan Gawel and Rebecca Riske for their statistical support. We thank Rebecca Plant, MSc, from QXV Communications (Macclesfield, UK), an Ashfield company, part of UDG Healthcare plc, who provided medical writing support funded by MedImmune in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3). This study was sponsored by MedImmune/AstraZeneca and Abbott Laboratories. Development of the ARCHITECT DPP-4 Immunoassay by Abbott Laboratories was funded by MedImmune/AstraZeneca. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Acknowledgments
Conflict of interest
PMH is an employee of Abbott, and holds stock or options in Abbott and AbbVie. NMJ is currently an employee of Abbott, and holds stock or options in Abbott. TR is currently an employee of Abbott. SEB is currently an employee of Abbott. MJD is currently an employee of Abbott. YX is currently an employee of Abbott. TSM is currently an employee of MedImmune, and holds stock or options in MedImmune. IV is currently an employee of MedImmune, and holds stock or options in AstraZeneca. ML is currently an employee of MedImmune, and holds stock or options in AstraZeneca. XX is currently an employee of Bristol-Myers Squibb, and was an employee of MedImmune when this work was completed. PSC is currently an employee of Sanofi, and has previously sat on the board or been an advisory committee member of CHI. CYC is currently an employee of MedImmune. KS is currently an employee of MedImmune, and holds stock or options in AstraZeneca. LG is currently an employee of MedImmune, and holds stock or options in AstraZeneca. KR is currently an employee of MedImmune, is a named inventor on a pending patent application (International Patent Application No. PCT/US2015/12885) assigned to MedImmune, and holds stock or options in AstraZeneca. GJD is currently an employee of Abbott.
Role of the funding source
MedImmune contributed to the interpretation of the data, report writing, and funding of medical writing and editorial support. All authors had full access to data and contributed to data analyses, data interpretation and the report writing.
Contributor Information
Philip M. Hemken, Email: philip.hemken@abbott.com.
Nicolette M. Jeanblanc, Email: nicolette.jeanblanc@abbott.com.
Tracey Rae, Email: Tracey.Rae@abbott.com.
Susan E. Brophy, Email: susan.brophy@abbott.com.
Maria J. Datwyler, Email: maria.datwyler@abbott.com.
Ying Xu, Email: ying.xu1@abbott.com.
T. Scott Manetz, Email: ManetzS@medimmune.com.
Inna Vainshtein, Email: vainshteini@medimmune.com.
Meina Liang, Email: LiangM@medimmune.com.
Xiaodong Xiao, Email: xiaodong.xiao@bms.com.
Partha S. Chowdhury, Email: Partha.Chowdhury@sanofi.com.
Chien-ying Chang, Email: changc@medimmune.com.
Katie Streicher, Email: StreicherK@medimmune.com.
Lydia Greenlees, Email: GreenleesL@medimmune.com.
Koustubh Ranade, Email: RanadeK@medimmune.com.
Gerard J. Davis, Email: gerard.davis@abbott.com.
References
- 1.Zhu Z., Homer R.J., Wang Z., Chen Q., Geba G.P., Wang J. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J. Clin. Investig. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotsimbos T.C., Ernst P., Hamid Q.A. Interleukin-13 and interleukin-4 are coexpressed in atopic asthma. Proc. Assoc. Am. Phys. 1996;108:368–373. [PubMed] [Google Scholar]
- 3.Humbert M., Durham S.R., Kimmitt P., Powell N., Assoufi B., Pfister R. Elevated expression of messenger ribonucleic acid encoding IL-13 in the bronchial mucosa of atopic and nonatopic subjects with asthma. J. Allergy Clin. Immunol. 1997;99:657–665. doi: 10.1016/s0091-6749(97)70028-9. [DOI] [PubMed] [Google Scholar]
- 4.Naseer T., Minshall E.M., Leung D.Y., Laberge S., Ernst P., Martin R.J. Expression of IL-12 and IL-13 mRNA in asthma and their modulation in response to steroid therapy. Am. J. Respir. Crit. Care Med. 1997;155:845–851. doi: 10.1164/ajrccm.155.3.9117015. [DOI] [PubMed] [Google Scholar]
- 5.Komai-Koma M., McKay A., Thomson L., McSharry C., Chalmers G.W., Liew F.Y. Immuno-regulatory cytokines in asthma: IL-15 and IL-13 in induced sputum. Clin. Exp. Allergy. 2001;31:1441–1448. doi: 10.1046/j.1365-2222.2001.01174.x. [DOI] [PubMed] [Google Scholar]
- 6.Saha S.K., Berry M.A., Parker D., Siddiqui S., Morgan A., May R. Increased sputum and bronchial biopsy IL-13 expression in severe asthma. J. Allergy Clin. Immunol. 2008;121:685–691. doi: 10.1016/j.jaci.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arron J.R., Choy D.F., Scheerens H., Matthews J.G. Noninvasive biomarkers that predict treatment benefit from biologic therapies in asthma. Ann. Am. Thorac. Soc. 2013;10:Suppl:S206–Suppl:S213. doi: 10.1513/AnnalsATS.201303-047AW. [DOI] [PubMed] [Google Scholar]
- 8.Brightling C., Wang M., Braddock M., Nordenmark L., Gottlow M., Colice G. MESOS: considerations in designing a mechanistic study for a biologic used to treat asthma. Clin. Investig. 2015;5:713–722. [Google Scholar]
- 9.Busse W.W., Wang M., Gibson J., Gottlow M., Braddock M., Colice G. TROPOS: designing a clinical trial to evaluate the oral corticosteroid-sparing effect of a biologic in severe asthma. Clin. Investig. 2015;5:723–730. [Google Scholar]
- 10.Panettieri R.A., Jr., Brightling C., Sjobring U., Péterffy A., Tornling G., Daoud S.Z. STRATOS 1 and 2: considerations in clinical trial design for a fully human monoclonal antibody in severe asthma. Clin. Investig. 2015;5:701–711. [Google Scholar]
- 11.St Ledger K., Agee S.J., Kasaian M.T., Forlow S.B., Durn B.L., Minyard J. Analytical validation of a highly sensitive microparticle-based immunoassay for the quantitation of IL-13 in human serum using the Erenna immunoassay system. J. Immunol. Methods. 2009;350:161–170. doi: 10.1016/j.jim.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Poole A., Urbanek C., Eng C., Schageman J., Jacobson S., O'Connor B.P. Dissecting childhood asthma with nasal transcriptomics distinguishes subphenotypes of disease. J. Allergy Clin. Immunol. 2014;133:670–678. doi: 10.1016/j.jaci.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ranade K., Pham T.-H., Damera G., Brohawn P., Pilataxi F., Kuziora M. Dipeptidyl peptidase-4 (dpp-4) is a novel predictive biomarker for the investigational anti-Il-13 targeted therapy tralokinumab. Am. J. Respir. Crit. Care Med. 2016:A4332. [Google Scholar]
- 14.Brightling C.E., Chanez P., Leigh R., O'Byrne P.M., Korn S., She D. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2015;3:692–701. doi: 10.1016/S2213-2600(15)00197-6. [DOI] [PubMed] [Google Scholar]
- 15.Wagner L., Klemann C., Stephan M., von Horsten S. Unravelling the immunological roles of dipeptidyl peptidase 4 (DPP4) activity and/or structure homologue (DASH) proteins. Clin. Exp. Immunol. 2016;184:265–283. doi: 10.1111/cei.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drucker D.J. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care. 2007;30:1335–1343. doi: 10.2337/dc07-0228. [DOI] [PubMed] [Google Scholar]
- 17.Ussher J.R., Drucker D.J. Cardiovascular biology of the incretin system. Endocr. Rev. 2012;33:187–215. doi: 10.1210/er.2011-1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorrell M.D. Dipeptidyl peptidase IV and related enzymes in cell biology and liver disorders. Clin. Sci. 2005;108:277–292. doi: 10.1042/CS20040302. [DOI] [PubMed] [Google Scholar]
- 19.Yazbeck R., Howarth G.S., Abbott C.A. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol. Sci. 2009;30:600–607. doi: 10.1016/j.tips.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Pro B., Dang N.H. CD26/dipeptidyl peptidase IV and its role in cancer. Histol. Histopathol. 2004;19:1345–1351. doi: 10.14670/HH-19.1345. [DOI] [PubMed] [Google Scholar]
- 21.Cro L., Morabito F., Zucal N., Fabris S., Lionetti M., Cutrona G. CD26 expression in mature B-cell neoplasia: its possible role as a new prognostic marker in B-CLL. Hematol. Oncol. 2009;27:140–147. doi: 10.1002/hon.888. [DOI] [PubMed] [Google Scholar]
- 22.Quinn F.A. Architect® i2000® and i2000SR® analyzers. In: Wild D., editor. The Immunoassay Handbook. 3rd ed. Elsevier Ltd; Amsterdam, Netherlands: 2005. [Google Scholar]
- 23.Jeanblanc N.M., Hemken P.M., Datwyler M.J., Brophy S.E., Manetz T.S., Lee R. Development of a new architect automated periostin immunoassay. Clin. Chim. Acta. 2016 doi: 10.1016/j.cca.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 24.P. Gagnon, Purification Tools for Monoclonal Antibodies. Tuscon, AZ: Validated Biosystems, 1996: 155–90, 99–202.
- 25.CLSI., Evaluation of precision performance of quantitative measurement methods; approved guideline. CLSI Document EP05-A2. 2nd ed. CLSI, Wayne, PA, 2004.
- 26.CLSI., Evaluation of the linearity of quantitative measurement procedures: a statistical approach; approved guideline. CLSI Document EP06-A. CLSI, Wayne, PA, 2003.
- 27.Christofides N.D. Free analyte immunoassay. In: Wild D., editor. The Immunoassay handbook. 2nd ed. Nature Publishing Group; London, UK: 2001. pp. 61–77. [Google Scholar]
- 28.CLSI., Interference testing in clinical chemistry; approved guideline. CLSI Document EP07-A2. 2nd ed. CLSI, Wayne, PA, 2005.
- 29.CLSI., Evaluation of detection capability for clinical laboratory measurement procedures; approved guideline. CLSI Document EP17-A2. 2nd ed. CLSI, Wayne, PA, 2012.
- 30.Tate J., Ward G. Interferences in immunoassay. Clin. Biochem. Rev. 2004;25:105–120. [PMC free article] [PubMed] [Google Scholar]