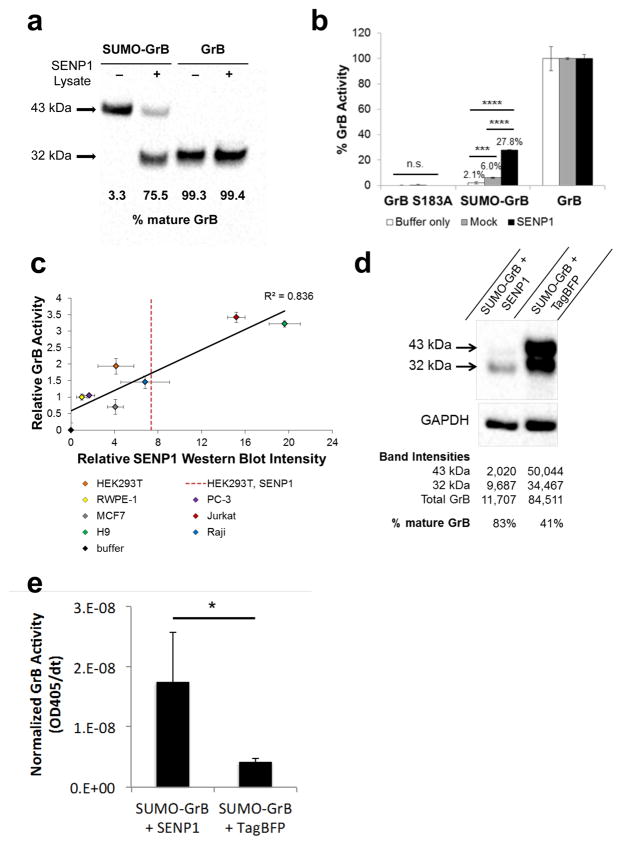
Figure 2.
SUMO-GrB is efficiently processed and activated by SENP1 in a dose-responsive manner. (a) Cleavage of purified SUMO-GrB following co-incubation with mock or SENP1-overexpressing HEK293T lysates. Percent mature GrB was calculated by normalizing the intensity of the 32-kDa mature GrB band by the total intensity of the 43-kDa SUMO-GrB and the mature GrB bands on the western blot. Purified mature GrB was included as a control, which was unaffected by the presence of SENP1. (b) Enzymatic activity of GrB molecules as quantified by Ac-IEPD-pNA cleavage. Purified SUMO-GrB was co-incubated with mock or SENP1-overexpressing HEK293T lysates as in (a). Percent GrB activity was calculated as the rate of Ac-IEPD-pNA cleavage normalized to the rate of cleavage by mature GrB. (c) SENP1 dose-responsive activation of purified SUMO-GrB following co-incubation with lysates from a panel of seven human cell lines. SENP1 protein level in each cell line was quantified via western blot, and GrB activity was quantified via the Ac-IEPD-pNA cleavage assay. GrB activity and SENP1 protein level for each sample were normalized to those of RWPE-1 to enable clear visualization of the data spread. The red dotted line indicates the SENP1 expression level of HEK293T cells transiently transfected with SENP1-encoding plasmids. Western blots corresponding to x-axis values are shown in Figure S1, Supporting Information. (d) Intracellular cleavage of SUMO-GrB by SENP1. HEK293T cells were transiently transfected with SUMO-GrB plus plasmids encoding either TagBFP-T2A-SENP1 or TagBFP alone. Cell lysates were collected 24 hours post transfection and probed for GrB by western blot. Total GrB refers to the sum of the 43-kDa SUMO-GrB and 32-kDa mature GrB bands, and % mature GrB was calculated as described in (a). (e) Enzymatic activity of SUMO-GrB–transfected cell lysates as quantified by Ac-IEPD-pNA cleavage. Twenty-five μg of the same cell lysates as shown in (d) were reacted with 200 μM Ac-IEPD-pNA. The rate of Ac-IEPD-pNA cleavage was normalized by the amount of total GrB in each lysate based on western blot results shown in (d). All plotted values indicate the mean of triplicate samples and error bars represent ±1 standard deviation (s.d.). * p < 5E–3; ***p < 5E–5; ****p < 5E–12.