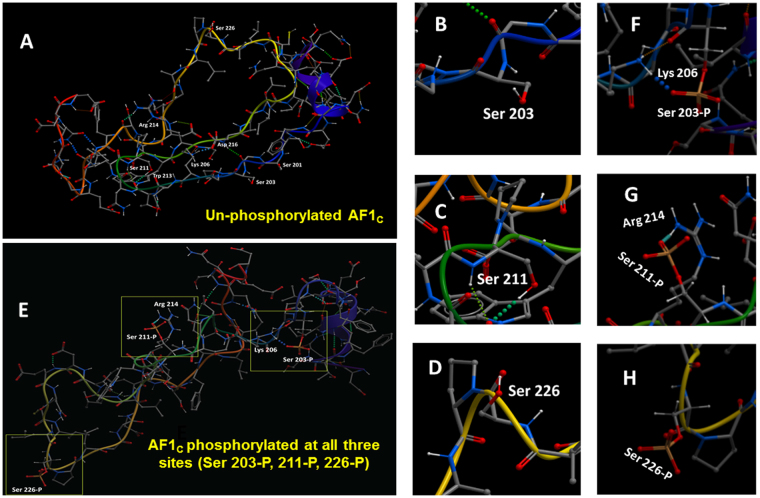
Figure 1.
Simultaneous phosphorylation of all three sites (Ser203, 211 & 226) in the GR results in conformational changes in AF1. (A) Representative low-energy conformations of the non-phosphorylated AF1C peptide. (B–D) close up view of non-phosphorylated Ser203, 211 & 226 residues, respectively. (E) Representative low-energy conformations of the AF1C peptide when all three sites (Ser203, 211 & 226) are simultaneously phosphorylated. (F–H) close up view of phosphorylated Ser203, 211 & 226 residues and their respective binding to nearby residues. Phosphorylated Ser203 and 211 are shown forming a hydrogen bond network that is displayed as dots between donor and acceptor atoms.