Abstract
Purpose:
Despite guidelines emphasizing symptom management over aggressive treatment, end-of-life care for persons with cancer in the United States is highly variable. In consultation with a regional collaboration of patients, providers, and payers, we investigated indicators of high-quality end-of-life care to describe patterns of care, identify areas for improvement, and inform future interventions to enhance end-of-life care for patients with cancer.
Methods:
We linked insurance claims to clinical information from the western Washington SEER database. We included persons ≥ 18 years of age who had been diagnosed with an invasive solid tumor between January 1, 2007, and December 31, 2015, and who had a recorded death date, were enrolled in a commercial plan for the last month of life, and made at least one insurance claim in the last 90 days of life.
Results:
In the last month of life, among 6,568 commercially insured patients, 56.3% were hospitalized and 48.6% underwent at least one imaging scan. Among patients younger than 65 years of age, 31.4% were enrolled in hospice; of those younger than 65 years of age who were not enrolled in hospice, 40.5% had received an opioid prescription. Over time, opioid use in the last 30 days of life among young adults not enrolled in hospice dropped from 44.7% in the period 2007 to 2009 to 42.5% in the period 2010 to 2012 and to 36.7% in the period 2013 to 2015.
Conclusion:
Hospitalization and high-cost imaging scans are burdensome to patients and caregivers at the end of life. Our findings suggest that policies that facilitate appropriate imaging, opioid, and hospice use and that encourage supportive care may improve end-of-life care and quality of life.
INTRODUCTION
During the last few weeks or months of life of a patient with cancer, clinical guidelines recommend changes in patient management, with less focus on aggressive cancer treatment and more focus on relieving symptoms and clarifying goals of care.1 Studies suggest that a lower intensity of care at the end of life is associated with a higher quality of life for patients and higher satisfaction with care among family members.2 Despite such guidelines, end-of-life care for persons with cancer in the United States is highly variable in intensity.3
Improving cancer care delivery at end of life may decrease the burden for patients and families as they avoid costly interventions that do not increase the length or quality of life. One study found that patients who avoid hospitalization have the highest quality of life at the end of life,4 whereas chemotherapy near death is associated with decreased quality of life and a reduction in performance status among healthier patients.5
To better understand ways to improve cancer care delivery and value in cancer care, we established a regional consortium of patients, clinicians, researchers, and health plan representatives. With our consortium partners, we created a database linking commercial insurance claims to cancer registry and death records. In conjunction with consortium stakeholder input, published literature,6,7 and society guidelines,8,9 we identified high-priority quality indicators for palliative and end-of-life care. Data on care patterns for commercially insured adults are needed, because previous studies on end-of-life care in the United States have focused largely on the Medicare population. Studies describing care patterns for patients with cancer who are younger than 65 years of age have focused on Medicaid recipients,10 enrollees in a single integrated delivery system,11,12 or commercially insured patients at a single tertiary center.13 Using this unique database, we describe patterns of care in this population of commercially insured adults in western Washington State and identify areas for care delivery enhancement and future interventions to improve end-of-life care for patients with cancer.
METHODS
Setting and Study Population
Fred Hutchinson Cancer Research Center investigators conducted this study with leaders at Regence Blue Shield and Premera Blue Cross, two not-for-profit commercial insurers. We linked health plan enrollment files to cancer registry records from the Western Washington Cancer Surveillance System (CSS). As part of the National Cancer Institute’s SEER registry, the CSS collects comprehensive information on staging, initial treatment, and survival for persons diagnosed with malignancies in western Washington.14 The linked database includes 80,377 persons ≥ 18 years of age who were diagnosed with cancer between January 1, 2007, and December 31, 2015, representing 37% of people with cancer as recorded by the CSS registry during the same time period.
For this study, we included individuals who had a known date of cancer diagnosis, a solid tumor, and a non–in situ diagnosis, and who were not diagnosed on the day of their death. After linkage of CSS and insurance enrollment records, we extracted claims for inpatient stays, outpatient visits, and pharmacy claims. We restricted our study population to patients with cancer with a recorded death date who made at least one claim in their last 90 days of life and who were enrolled continuously in their insurance plan during their last month of life.
Defining and Identifying Recommended and Undesirable End-of-Life Services
We convened a multistakeholder panel consisting of community and academic clinicians, health insurance executives, and patient advocates to establish the most salient measures of interest. The group reviewed services that are recommended at end of life (eg, use of pain medications, hospice admission) as well as those that are not recommended (eg, advanced imaging scans) or are considered not desirable (eg, emergency department [ED] visits, hospitalizations).15 Appendix Table A1 (online only) contains the final list of metrics and the cor-responding evaluation and management codes used to identify specific services and events.
Hospice services include both inpatient and outpatient care and were identified using procedure, revenue center, and place of service codes. To restrict the measure to patients who enrolled in hospice, rather than those who only had a hospice consult, patients were required to have two hospice claims on different days. Once a patient was flagged with two hospice claims, he or she was considered to be in hospice continuously until death. Because hospice claims were unavailable for all patients older than 65 years of age, we excluded these patients from our hospice analysis. We report opioid use in the last month of life for those younger than 65 years of age who were not enrolled in hospice, because pharmacy claims were unavailable after hospice enrollment and for some patients older than 65 years of age.
Place of death was determined by the location of service for claims on the day of and the day before the day of death, as recorded in the cancer registry. Codes on the claims for inpatient service, ED, nursing home, and hospice were identified for each patient. If multiple locations of service were captured, then the patient was assigned to the location with the highest intensity of care, in the following order, from most to least intense: (1) inpatient, (2) ED, (3) nursing home, and (4) hospice. Patients with no claims or only outpatient claims where classified as Other/Home. Because claims on the day of death were not available for all patients older than 65 years of age, we report the place of death for enrollees who were 18 to 64 years of age.
We created separate groups for patients with the most commonly occurring cancers in our sample, including breast, lung, colorectal, and pancreatic cancers. We grouped cervical, uterine, ovarian, and vaginal cancers in one group as gynecologic malignancy. Given the small number of patients in each group and the small number of decedents in the case of prostate cancer, we categorized the remaining solid tumor cancer diagnoses of bladder, head and neck, kidney, liver, melanoma, prostate, and thyroid under the category of other.
Statistical Analysis
We performed a descriptive analysis of the demographics of the population and determined the percentage of patients receiving recommended or less desirable end-of-life health care services. We constructed multivariate logistic regression models to evaluate the association between health care use at the end of life and demographic factors such as age, sex, ethnicity, and marital status. We fit a logistic regression model for chemotherapy use in the last 14 days of life and each of the following outcomes in the last 30 days of life: hospitalization, ED visit, any imaging receipt, radiation, chemotherapy, hospice enrollment, and use of opioid medication. Imaging receipt included computed tomography (CT) scan, magnetic resonance imaging (MRI) scan, positron emission tomography (PET) scan, and bone scan. Because we included patients in our sample who had lived for < 30 days after diagnosis, in each regression we controlled for the number of days observed between cancer diagnosis and death. We measured trends in health care use over three time periods (2007 to 2009, 2010 to 2012, and 2013 to 2015) and performed a Cochrane-Armitage test for trend. We used Stata version 14.1 (STATA, College Station, TX) for all statistical analyses.
The Fred Hutchinson Cancer Research Center Institutional Review Board approved this study.
RESULTS
The linkage of Western Washington SEER cancer registry and insurance enrollment files identified 80,377 unique individuals with cancer who were ≥ 18 years of age at the time of diagnosis, 395 of whom were later excluded because of a lack of diagnosis date, diagnosis at autopsy, diagnosis via death certificate, or death on the day of diagnosis. We excluded 65,272 patients who did not die during the study period, 1,783 persons without an invasive solid tumor, and 6,359 persons who were not enrolled in either insurance plan for their last month of life or did not make a claim in the last 90 days of life. We included 6,568 persons in our final analysis.
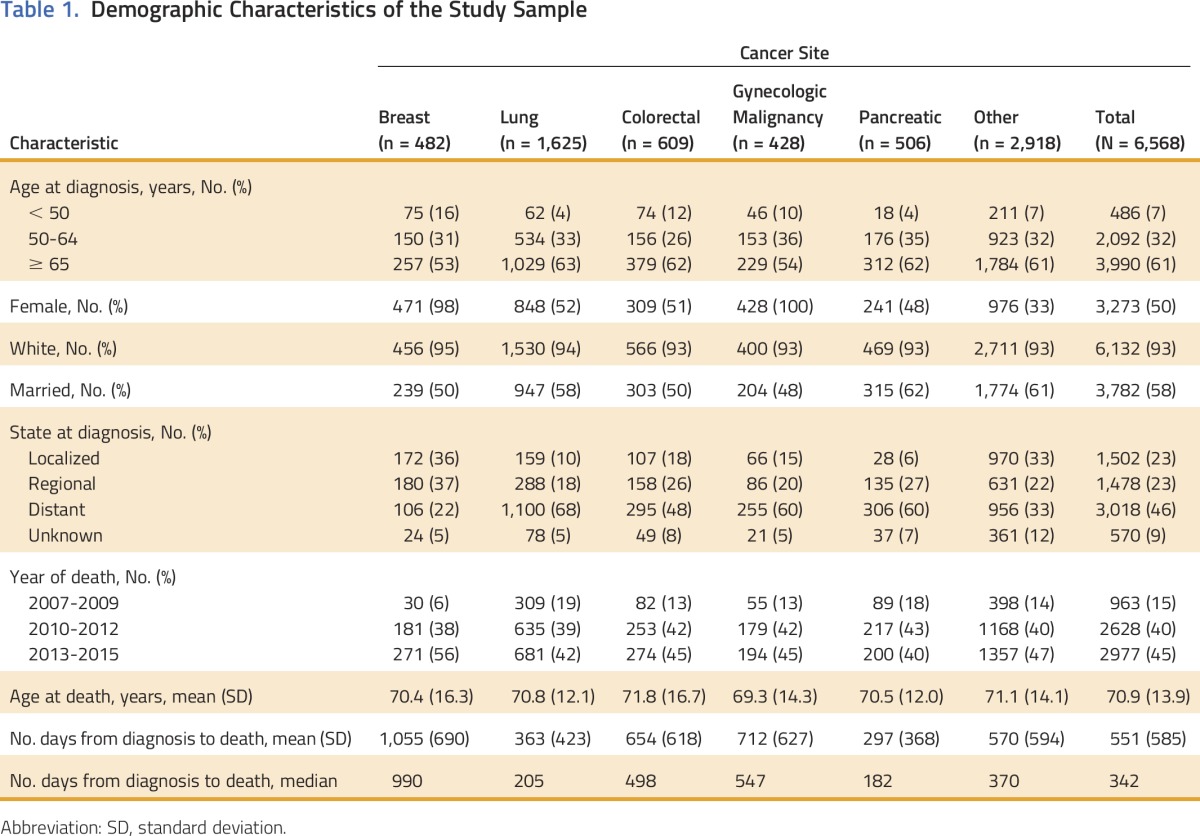
Table 1 lists the demographic characteristics of this commercially insured population, both overall and by cancer type. Of 6,568 people, 61% were ≥ 65 years of age, 50% were female, 93% were white, and 58% were married at the time of diagnosis. Approximately one half of patients (46%) were diagnosed with metastatic disease; this varied from 22% of patients with breast cancer to 68% of patients with lung cancer. Across all cancers, median survival was 342 days after diagnosis. We observed the lowest median survival after diagnosis among patients with pancreatic cancer (182 days) and the highest among patients with breast cancer (990 days).
Table 1.
Demographic Characteristics of the Study Sample
Patterns of Care
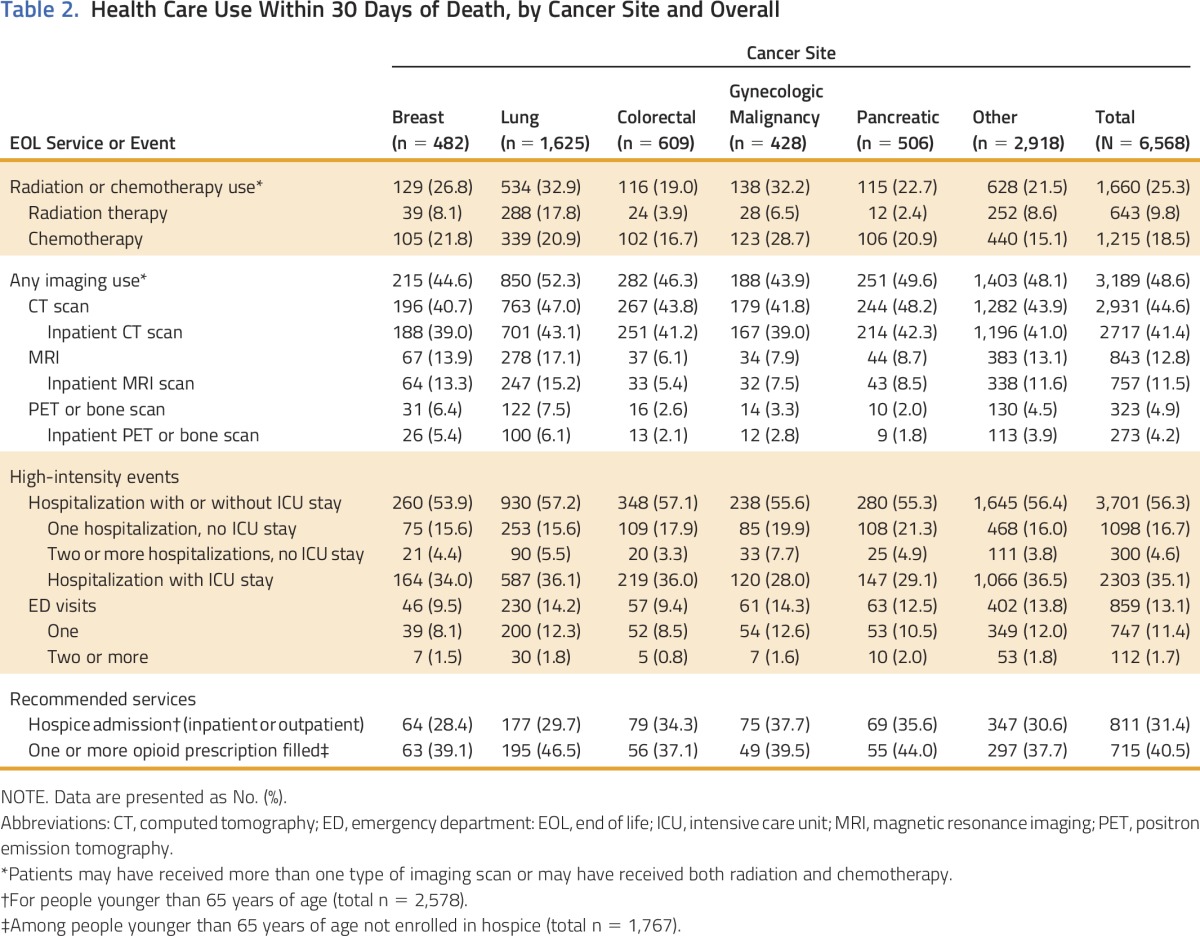
In Table 2, we present health care use during the last month of life, overall and by specific cancer type. During their last 30 days, more than one half of patients (56.3%) were hospitalized, whereas 48.6% received at least one advanced imaging scan. Most scans were CT scans (44.6% of participants); 12.8% of participants underwent an MRI scan, and 4.9% underwent PET or a bone scan. Across all imaging types, the majority of scans occurred in inpatient settings. Almost one fifth of patients received chemotherapy and/or radiation in their last 30 days; 18.5% of patients received chemotherapy, whereas 9.8% received radiation. During the last 14 days of life, 512 patients (7.8%) received chemotherapy. Less than one third of patients (31.4%) younger than 65 years of age were enrolled in hospice for the last 30 days of life. Of those younger than 65 years of age who were not enrolled in hospice, 40.5% received an opioid prescription in their last month of life.
Table 2.
Health Care Use Within 30 Days of Death, by Cancer Site and Overall
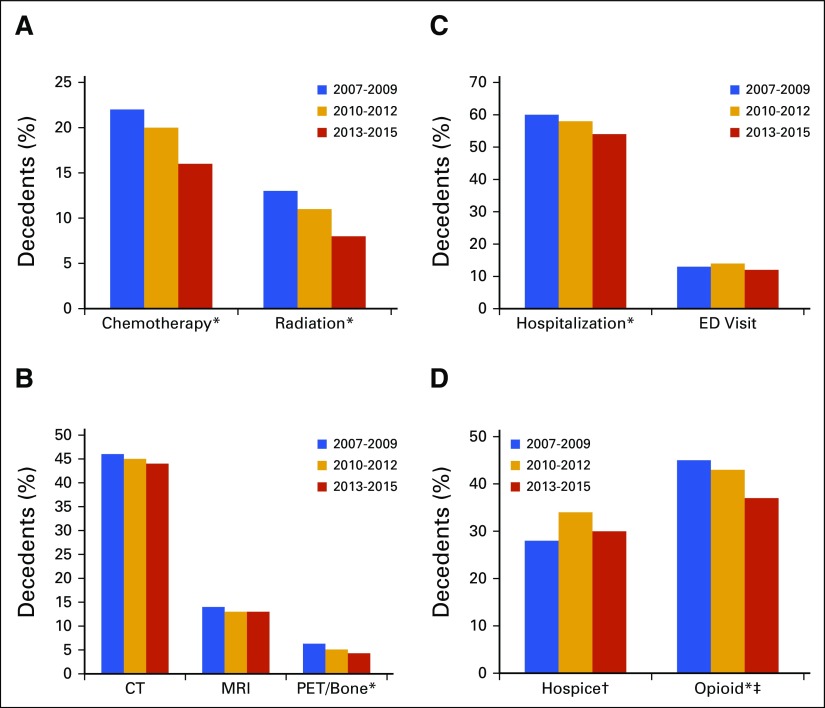
Use Trends Over Time
Figure 1 notes trends in health care use over the last 30 days of life for the different years of this study. We report the significance of Cochrane-Armitage tests for trend together with each finding. Hospice use among patients younger than 65 years of age increased from 27.5% in the period 2007 to 2009 to 34.1% in the period 2010 to 2012, before dropping to 30.4% in the period 2013 to 2015 (P = .75). Over time, opioid use dropped from 44.7% in the period 2007 to 2009 to 42.5% in the period 2010 to 2012 and to 36.7% in the period 2013 to 2015 (P < .01).
Fig 1.
Trends in health care use in the last 30 days of life by year of death and percentage of decedents: (A) chemotherapy or radiation use, (B) imaging use, (C) hospitalization and ED visits, (D) hospice and opioid use. Overall, 963 patients died between 2007 and 2009, 2,628 between 2010 and 2012, and 2,977 between 2013 and 2015. (*) Statistically significant trend over time. (†) For people under 65 years of age: 426 died between 2007 and 2009, 1,088 died between 2010 and 2012, and 1,064 died between 2013 and 2015. (‡) Under 65 years, not enrolled in hospice. CT, computed tomography; ED, emergency department; MRI, magnetic resonance imaging; PET, positron emission tomography.
Among all patients, ED visits in the last 30 days of life peaked at 14.1% in the 2010 to 2012 period after starting at 12.9% in the 2007 to 2009 period, then dropped to 12.3% in the 2013 to 2015 period (P = .25). Hospitalization in the last month of life decreased over time, from 60.3% to 57.5% and then to 54% (P < .01). Chemotherapy use also declined, from 22.2% to 20.2% and then to 15.8% in the last 30 days of life (P < .01) and from 10.5% to 8.3% and then to 6.5% in the last 2 weeks of life (P < .01). We found a significant decrease in radiation receipt in the last month of life, from 13% to 11% and then to 8% across the three time periods (P < .01). We found no significance in trends in CT (P = .24) or MRI (P = .31) use over time; however, the trend in PET and bone scans was significant (P < .01).
Location of Death
Among 2,578 decedents younger than 65 years of age, the locations at death were as follows: hospice (40.1%), hospital inpatient (33.3%), home or other (22.0%), nursing home (2.5%), and ED (2.1%). The highest percentage of patients dying in hospice care (48.2%) was among women with a gynecologic malignancy; this group also had the lowest percentage of patients dying in the hospital (24.6%). Patients with breast cancer were the most likely to die in the hospital (41.3%) and the least likely to die in hospice care (32.4%). Among patients with pancreatic, colorectal, and lung cancers, the percentages dying in hospice were smaller, at 45.9%, 42.2%, and 38.4%, respectively, and the percentages dying in inpatient settings were 30.4%, 31.7%, and 35.1%, respectively.
Logistic Regression
Adjusting for type of cancer, we found that female sex (odds ratio [OR], 0.79; 95% CI, 0.70 to 0.89) was significantly associated with lower odds of inpatient hospitalization. We found no association between ED use at the end of life and any demographic variables including sex, ethnicity, age, and marital status.
Among enrollees younger than 65 years of age, women (OR, 0.73; 95% CI, 0.61 to 0.87) were 27% less likely to receive opioids in the last 30 days of life compared with men. Patients of nonwhite ethnicity were statistically less likely to enroll in hospice (OR, 0.60; 95% CI, 0.44 to 0.82) or to receive opioids (OR, 0.54; 95% CI, 0.40 to 0.72) compared with white patients.
Female patients (OR, 0.85; 95% CI, 0.76 to 0.96) and nonmarried individuals (OR, 0.87; 95% CI, 0.78 to 0.97) were less likely to receive any imaging scans in their last 30 days of life. Unmarried patients were less likely to receive chemotherapy (OR, 0.71; 95% CI, 0.62 to 0.82) in the last 30 days or last 14 days of life (OR, 0.68; 95% CI, 0.56 to 0.83). Each 1 year increase in age was associated with a reduced likelihood of receiving radiation (OR, 0.82; 95% CI, 0.68 to 0.99).
DISCUSSION
In our cohort of commercially insured adults, we found that a majority of patients received imaging scans, radiation, or chemotherapy in the last 30 days of life, and almost one half experienced a hospitalization or emergency room visit. This high observed use may be, in part, a result of insurance structure and incentives, because our study population is composed of people with fee-for-service commercial insurance. As previous studies have noted, when compared with patients in a health maintenance organization or capitation model, those with fee-for-service insurance have higher-intensity health care use at the end of life.16,17
Many of our findings align with those of previous studies, because we also note that men with cancer are more likely to experience hospitalization in the last month of life compared with women.18 Of patients younger than 65 years of age who were not enrolled in hospice, 40.5% received a prescription for an opioid medication, similar to the findings of earlier studies.19 We found that 33% of younger patients died in the hospital, comparable to the 28% observed in a study of commercially insured patients with cancer who died between July 2010 and December 2013.13 Although chemotherapy use in the last days of life among Medicare enrollees has stayed steady over time at approximately 4.5%,20 we observed a downward trend over time. This may have been caused by increased acceptance of palliative care interventions or a reduced offering of chemotherapy by practitioners.
One third of patients younger than 65 years of age enrolled in hospice for the last month of life; other researchers noted 24% to 32% enrollment in hospice among Medicaid-enrolled adults 21 to 64 years of age who had been diagnosed with stage IV lung cancer between 2002 and 2006.10 This finding may be the result of patient and provider preferences for more aggressive interventions rather than palliative care for younger patients. In one study, despite a documented preference for comfort care, 75% of young adults with advanced cancer received aggressive end-of-life care.12 Among younger adults, palliative care is more likely to be initiated close to death,21 and if hospice is elected, stays are shorter22 compared with adults older than 65 years of age.
Almost one half of patients in this study (48.6%) received an advanced imaging scan. This percentage is higher than the 34.3% observed by Hu et al23 among Medicare beneficiaries with stage IV cancer diagnosed between 2002 and 2006. Unfortunately, because we did not have access to electronic medical records, we do not know the reasons these imaging scans were ordered, be it in response to new symptoms, to inform de-escalation of care, or to gauge therapy response.
We observed that 40.5% of enrollees younger than 65 years of age received opioids in their last 30 days. Because we did not have access to the medical records, we do not know if some patients did not experience pain or did not feel that their pain symptoms required opioid therapy. The observed low use of opioids may have been a result of patient reluctance caused by fear of addiction or the perceived stigma around opioid use,24 or a result of caregiver misunderstanding around the use of opioids at the end of life.25 Of note, the percentage of patients receiving opioids decreased each year from 2007 to 2015, with the largest percentage drop occurring after 2012. It is possible that the 2012 legalization of marijuana in Washington State reduced the stigma associated with use. The legalization of medical marijuana has been associated with a reduction in opioid-related hospitalizations,26 and the concurrent use of marijuana and opioids can result in a reduction in opioid use.27 Unfortunately, at this time, no study has documented patterns of marijuana use among patients with cancer in Washington State.
This study has limitations. First, without access to clinical notes, electronic medical records, or patient-reported outcomes, we could not evaluate patient, physician, or family or caregiver care preferences. Second, the time horizon for this study was before the enactment of newer current procedural terminology billing codes for palliative care consultations. The early provision of palliative care has been found to improve patient outcomes,28 and consultations are associated with reduced intensive care unit admissions during hospitalization.29 It is possible that palliative care consultations may have influenced some of the care patterns we observed, but we were unable to measure the impact. Finally, because we were unable to access hospice claims, outpatient medication use, or claims on the day of death for patients older than 65 years of age, we reported hospice use, opioid use, and place of death for patients younger than 65 years of age. Despite these limitations, our results suggest that problems with overuse of aggressive care and underuse of palliative and hospice services affected populations of younger patients with commercial insurance as well as Medicare enrollees and persisted through 2015.
Our findings identify areas for additional investigation to improve end-of-life care for patients with cancer. First, more than one half of patients experienced hospitalization or an ED visit in the last month of life, which may represent potentially avoidable health care encounters. Care models that emphasize care coordination and symptom management may help reduce the incidence of such visits. The observed high use of imaging may be for disease progression monitoring or to inform decisions around cancer-directed therapy. Studies that elucidate the reasons for such imaging and the impact of imaging scans on patient and caregiver quality of life are needed to inform reimbursement policies and guidelines that promote value-based, patient-centered care. Finally, fewer than one half of patients younger than 65 years of age who were not enrolled in hospice received opioids for pain relief, and only one third used hospice for the last month of life, indicating areas for potential improvement in end-of-life service provision to younger adults. Research to identify barriers to hospice use and supportive care in this population can inform interventions to ensure that patients do not have untreated pain and are provided services to maximize their quality of life.
ACKNOWLEDGMENT
Supported by unrestricted grants from Premera Blue Cross, Regence Blue Shield, Cambia Health Solutions, and the Fred Hutchinson Cancer Research Center, and by National Heart, Lung, and Blood Institute Grant No. T32 HL125195-02 (C.M.). Presented in part at the 2016 International Society for Pharmacoeconomics and Outcomes Research Annual Meeting, May 21-25, 2016, Washington, DC, and the 2016 ASCO Palliative Care in Oncology Symposium, September 9-10, 2016, in San Francisco, CA.
Appendix
Table A1.
List of Codes Used to Define Healthcare Use in the Last 30 Days of Life

AUTHOR CONTRIBUTIONS
Conception and design: Cara L. McDermott, Catherine Fedorenko, Bruce Smith, Ted Conklin, Scott D. Ramsey
Collection and assembly of data: Cara L. McDermott, Catherine Fedorenko, Karma Kreizenbeck, Bruce Smith, Ted Conklin, Scott D. Ramsey
Data analysis and interpretation: Cara L. McDermott, Qin Sun, J. Randall Curtis, Scott D. Ramsey
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
End-of-Life Services Among Patients With Cancer: Evidence From Cancer Registry Records Linked With Commercial Health Insurance Claims
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
Cara L. McDermott
No relationship to disclose
Catherine Fedorenko
No relationship to disclose
Karma Kreizenbeck
No relationship to disclose
Qin Sun
No relationship to disclose
Bruce Smith
No relationship to disclose
J. Randall Curtis
No relationship to disclose
Ted Conklin
Stock or Other Ownership: Carena (very small interest in privately held company that does virtual care)
Scott D. Ramsey
Consulting or Advisory Role: Bayer Schering Pharma, Bristol-Myers Squibb, Genentech
REFERENCES
- 1.Levy M, Smith T, Alvarez-Perez A, et al. : Palliative care version 1.2016. J Natl Compr Canc Netw 14:82-113, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Wright AA, Keating NL, Ayanian JZ, et al. : Family perspectives on aggressive cancer care near the end of life. JAMA 315:284-292, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morden NE, Chang CH, Jacobson JO, et al. : End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Health Aff (Millwood) 31:786-796, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang B, Nilsson ME, Prigerson HG: Factors important to patients’ quality of life at the end of life. Arch Intern Med 172:1133-1142, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prigerson HG, Bao Y, Shah MA, et al. : Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol 1:778-784, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Earle CC, Neville BA, Landrum MB, et al. : Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol 22:315-321, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Earle CC, Neville BA, Landrum MB, et al. : Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int J Qual Health Care 17:505-509, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Institute of Medicine: Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. National Academies Press, Washington, DC, 2015. [PubMed] [Google Scholar]
- 9.Smith TJ, Temin S, Alesi ER, et al. : American Society of Clinical Oncology provisional clinical opinion: The integration of palliative care into standard oncology care. J Clin Oncol 30:880-887, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Mack JW, Chen K, Boscoe FP, et al. : Underuse of hospice care by Medicaid-insured patients with stage IV lung cancer in New York and California. J Clin Oncol 31:2569-2579, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mack JW, Chen LH, Cannavale K, et al. : End-of-life care intensity among adolescent and young adult patients with cancer in Kaiser Permanente Southern California. JAMA Oncol 1:592-600, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mack JW, Cannavale K, Sattayapiwat O, et al. : Care in the final month of life among adolescent and young adult cancer patients in Kaiser Permanente Southern California. J Palliat Med 19:1136-1141, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks GA, Stuver SO, Zhang Y, et al. : Characteristics associated with in-hospital death among commercially insured decedents with cancer. J Palliat Med 20:42-47, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Fred Hutchinson Cancer Research Center : About the Cancer Surveillance System. Seattle, WA, Cancer Surveillance System, 2014 [Google Scholar]
- 15.Earle CC, Landrum MB, Souza JM, et al. : Aggressiveness of cancer care near the end of life: Is it a quality-of-care issue? J Clin Oncol 26:3860-3866, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howard M, Chalifoux M, Tanuseputro P: Does primary care model effect healthcare at the end of life? A population-based retrospective cohort Study. J Palliat Med 20:344-351, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Stevenson DG, Ayanian JZ, Zaslavsky AM, et al. : Service use at the end-of-life in Medicare advantage versus traditional Medicare. Med Care 51:931-937, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma RK, Prigerson HG, Penedo FJ, et al. : Male-female patient differences in the association between end-of-life discussions and receipt of intensive care near death. Cancer 121:2814-2820, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higginson IJ, Gao W: Opioid prescribing for cancer pain during the last 3 months of life: Associated factors and 9-year trends in a nationwide United Kingdom cohort study. J Clin Oncol 30:4373-4379, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Wang SY, Hall J, Pollack CE, et al. : Trends in end-of-life cancer care in the Medicare program. J Geriatr Oncol 7:116-125, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keim-Malpass J, Erickson JM, Malpass HC: End-of-life care characteristics for young adults with cancer who die in the hospital. J Palliat Med 17:1359-1364, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Connor NR, Hu R, Harris PS, et al. : Hospice admissions for cancer in the final days of life: Independent predictors and implications for quality measures. J Clin Oncol 32:3184-3189, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu YY, Kwok AC, Jiang W, et al. : High-cost imaging in elderly patients with stage IV cancer. J Natl Cancer Inst 104:1164-1172, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schumacher KL, West C, Dodd M, et al. : Pain management autobiographies and reluctance to use opioids for cancer pain management. Cancer Nurs 25:125-133, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Chi NC, Demiris G: Family caregivers’ pain management in end-of-life care: A systematic review. Am J Hosp Palliat CareCare 34:470-485, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Shi Y: Medical marijuana policies and hospitalizations related to marijuana and opioid pain reliever. Drug Alcohol Depend 173:144-150, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maguire DR, France CP: Impact of efficacy at the μ-opioid receptor on antinociceptive effects of combinations of μ-opioid receptor agonists and cannabinoid receptor agonists. J Pharmacol Exp Ther 351:383-389, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363:733-742, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Wallace SK, Waller DK, Tilley BC, et al. : Place of death among hospitalized patients with cancer at the end of life. J Palliat Med 18:667-676, 2015 [DOI] [PubMed] [Google Scholar]