Abstract
Notwithstanding the lack of exercise research, several reviews have championed the use of melatonin to combat metabolic syndrome. Therefore, this study compared substrate utilization during a 30-minute (min) graded exercise protocol following the ingestion of either 6 mg melatonin (M) or a placebo (P). Participants (12 women, 12 men) performed stages 1–5 of the Naughton graded exercise protocol (6 min per stage). The protocol was repeated 4 times (2x M, 2x P) at the same time of day with one week separating each session. Expired gases were monitored, VO2 and respiratory exchange ratio (RER) output was provided every 30s. Total, carbohydrate (CHO), and fat energy expenditures were obtained from the RER values using the formulae of Lusk. The VO2 at which CHO accounted for 50% of the total caloric expenditure was calculated by a VO2: RER regression line. Additionally, the energy derived was calculated by multiplying VO2 and the respective energy expenditures. Then, the total, CHO, and fat energies consumed during the 30 min of exercise were determined by calculating the area under the kJ/min: time curve using the trapezoid rule. The final data for the two similar trials were averaged and a paired-T test was used for statistical comparison. The average VO2 for 50% CHO usage was significantly lower following M (0.84 ± 0.54 l·min−1) than after P (1.21 ± 0.52 l·min−1). Also, average CHO kJ for M (627 ± 284) was significantly (p < 0.004) greater than P (504 ± 228), and accounted for a significantly greater contribution of total kJ consumed (M = 68% ±15 vs. P = 61% ± 18). Ingestion of melatonin 30 min prior to an aerobic exercise bout elevates CHO use during exercise.
Keywords: Carbohydrate metabolism, metabolic syndrome, cross-over point, caloric expenditure
INTRODUCTION
Metabolic syndrome clusters several metabolic abnormalities, including central (intra-abdominal) obesity, dyslipidemia (high cholesterol and blood lipids), hyperglycemia (high blood glucose), insulin resistance and hypertension (10, 21, 32). The ultimate importance of this cluster is to identify individuals at high risk for type 2 diabetes, cardiovascular disease, and strokes (10, 21, 32). Since metabolic syndrome is an interaction between several different disease states, numerous causative mechanisms leading to metabolic syndrome have been identified. The major factors identified include (but not limited to) inactivity, obesity, elevated circulating inflammatory and/or thrombotic markers (C-reactive protein, tumor necrosis factor-α (TNF-α), interleukin-6, and plasminogen activator inhibitor type 1), reduced anti-inflammatory molecules (adiponectin), excessive oxidative stress (too few anti-oxidants and/or too many reactive oxygen species), and growth hormone deficiency (10, 21, 32). In addition, recent evidence has emerged to suggest that alterations in circadian systems and sleep participate in the pathogenesis of the disease (24).
It is possible to prevent or delay the onset of metabolic syndrome by reducing lifestyle risk factors through moderate weight loss and increased physical activity (10, 12, 16, 21, 27). Several studies have shown that lifestyle changes that include exercise can significantly delay and possibly prevent this disease (12, 21, 27). Along with exercise, some experts have championed melatonin (8, 20, 22, 39) as a chemical that can possibly help with alleviating the symptoms of metabolic syndrome and its related diseases. Melatonin (N-acetyl-5-methoxytryptamine) is a hormone primarily produced by the pineal gland, but is also synthesized in the retina, kidneys, digestive tract, and leucocytes (1, 11, 22). Over the past ten years, melatonin has been the focus of considerable attention, albeit most of the attention is due to its function as a sleep aid or to improve circadian rhythm. Current internet search engine queries return numerous websites devoted to espousing the benefits of melatonin. In addition to its faddish appearance, mounting evidence in published in peer-reviewed scientific journals provides evidence of improving metabolic risk factors from exogenous melatonin (1, 8, 9, 16, 11, 20, 28, 38, 40). All of this research suggests that melatonin has crucial roles in various metabolic functions in addition to its role as an anti-oxidant (11, 20, 39), anti-inflammatory (1, 9, 16, 40), chronobiotic (4, 24), and growth hormone regulator (22, 26, 29).
Despite the numerous research studies on melatonin’s influence on the aforementioned facets of metabolic syndrome, the influence of exogenous melatonin upon the levels of blood glucose and lipids and their interplay with exercise have not been extensively studied, and inconsistent data have been published concerning melatonin’s influence on substrate utilization. For instance in animal studies, exogenous melatonin increases blood glucose in pigeons (17), but in rats melatonin can have either no influence on plasma glucose levels (18, 19) or it can cause increases in blood glucose (25, 35). In two studies where aerobic exercise to exhaustion was performed, muscle and liver glycogen content were significantly higher in melatonin-treated exercised animals compared to untreated exercised animals (25, 35). Also with acute aerobic exercise, plasma and liver lactate (18), plasma free fatty acid and plasma beta-hydroxybutyrate were significantly reduced (3, 19, 28). Melatonin-increased lipid utilization has also been reported in Japanese quail (41). In more germane studies, when mice were fed melatonin along with a high-fat diet, they had better control of blood glucose levels (37). Similarly, oral melatonin reduced fasting hyperglycemia in male Zucker diabetic fatty (ZDF) rats (2). Thus, it remains unclear how exogenous melatonin affects blood glucose or blood lipids and if melatonin’s effects upon these factors have a direct effect upon metabolic outcomes.
Similar to the animal research, the influence of exogenous melatonin on humans has not been extensively studied, and inconsistent data have been published. For instance, Peschke et al. (33) noted that during the night, diabetic patients have lower elevations in nighttime melatonin levels but higher blood glucose levels. The authors interpreted these occurrences as a possible relationship between low melatonin and hyperglycemia during sleep (33). On the other hand, Radziuk and Pye (34) showed that the nocturnal rise in blood glucose and endogenous glucose production seen in people with type 2 diabetes coincides with an increase in melatonin. In addition, two studies have shown promise for the use of melatonin as a therapeutic agent for Type 2 diabetes. In one study, Hussain et al. (15) gave melatonin and zinc acetate, alone or in combination with metformin. They found that when compared to a placebo, the aforementioned treatments improved fasting and postprandial glycemic control and lowered glycosylated hemoglobin (HbA1c) concentration (15). Additionally, Garfinkel et al. (13) found that long-term administration of prolonged-release melatonin resulted in lower HbA1c levels. In contrast to the two aforementioned studies, Cagnacci and associates (6) found that after ingesting melatonin, post-menopausal women had both reduced glucose usage and reduced insulin sensitivity. Additionally, a reduction in blood glucose clearance was seen following ingestion of melatonin (31). Changes in human exercise substrate utilization with exogenous melatonin, however, are poorly understood with only Sanders et al. (36) reporting that blood glucose levels during graded exercise were higher following the ingestion of melatonin.
Thus, notwithstanding the claims of different review articles (8, 20, 39), it would appear that exogenous melatonin usage to ameliorate metabolic syndrome is not wholly supported by human research. Unfortunately, information about the relationship in humans between melatonin and the most widely recommended therapeutic treatment, physical exercise, is still lacking. Therefore, the purpose of this research study was to further the information on melatonin by evaluating substrate utilization during graded exercise with and without exogenous melatonin. Since exercise intensity has an influence on substrate utilization, it was decided that the initial study would look at the carbohydrate and lipid utilization ‘crossover point’ (i.e. the workload where carbohydrate usage exceeds 50% of the total caloric consumption.
METHODS
Participants
Twelve males (24 ± 2.4 yrs; 176 ± 4.8 cm; 85.1 ± 17.8 kg) and twelve females (24 ± 1.1 yrs; 167 ± 7.4 cm; 63.6 ± 6.5 kg) who were enrolled in graduate Kinesiology classes at Louisiana State University (LSU) were recruited to participate in this project. All participants were healthy, non-smoking college students, and were not taking any medications. To be considered for this investigation, each participant had to have engaged in a minimum of 30 min of either resistive or endurance exercise (or both) 3 days per week for the preceding six months. The study was approved by the LSU institutional review board, and each subject submitted both written and oral consent before engaging in the experiment.
Protocol
A randomized balanced crossover design was used to test the acute effects of melatonin. Each participant was tested a total of four times after ingesting either 6 mg of melatonin (twice) or a placebo consisting of 50 mg of methylcellulose (twice). The experimental design was double-blind in addition to placebo controlled. One week separated each treatment, and all measurements were made at the same time of day. Prior to each visit, the subjects were asked to refrain from exercise for 24 h and to maintain their same dietary practices. All tests were performed before 11 am. Prior to each test, the subjects were asked to consume their normal breakfast one hour before their scheduled test time, and to eat that same breakfast prior to each test. Thirty minutes following the ingestion of melatonin or placebo, the substrate utilization tests began.
The participants reported to the laboratory one hour postprandial. One of the two treatment pills was ingested and the subject sat quietly for 30 minutes. After the rest period, the participants started walking on the treadmill using the Naughton protocol (30). The participants began at stage 1, and walked for six minutes at each stage, during which expiratory gases were collected using a Parvo Medics TrueOne 2400 metabolic cart (Sandy, UT, USA). This continued until five stages were completed (30 min).
The expired gases obtained during the graded exercise protocol were used to calculate oxygen consumption, carbon dioxide production, and respiratory exchange ratio (RER). To determine the ‘crossover point’, the VO2 (l·min−1) at which CHO accounted for 50% of the energy expended was calculated. To obtain this value, participants’ VO2 (l·min−1) values and the percentage of CHO being used (obtained from the RER using the formulae of Lusk (23)) for each 15s of exercise were plotted against each other (VO2 on the y-axis). A regression line was derived for this plot and the regression formulae were used to calculate the VO2 values when CHO use was at 50% of the total caloric expenditure.
In addition, the total calories from CHO and the relative percentage of CHO utilized during each six minute exercise stage were determined. This was calculated by plotting the product of VO2 (l·min−1) and the respective energy expenditure (total, and CHO kJ·min−1) for each 15s of exercise. The area under the kJ: time curve was then calculated using the trapezoid rule (14). The total and CHO calories utilized were obtained from the RER using the formulae of Lusk (23).
Statistical Analysis
The dependent variables analyzed were VO2 at 50% CHO as well total energy expended, total energy derived from CHO, and total relative energy derived from CHO (CHO * Total−1). For both the whole test (all 5 stages) and each individual stage, the values for the two tests performed with the same supplement were averaged and these averages were used for analysis. A paired T-test was used to determine if a difference existed between the melatonin and placebo conditions’ dependent variables. The level of significance was set at p < 0.05.
RESULTS
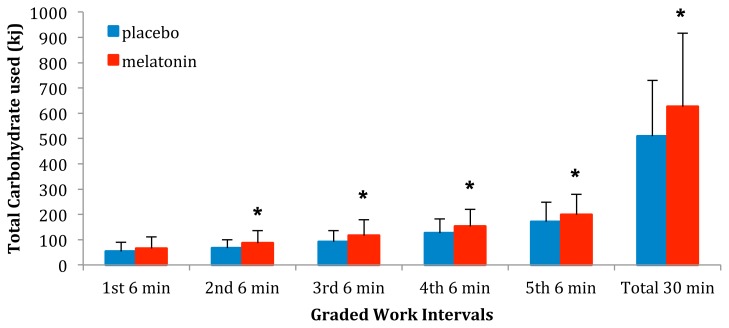
For the ‘crossover point’, there was a significant difference (p < 0.001) between the average VO2 (l·min−1) at which the participants achieved 50% CHO usage after P (1.21 ± 0.52 l·min−1) compared to M (0.84 ± 0.54 l·min−1). Average CHO expended (kJ) for all five stages as well as for the total 30 minutes are presented in Figure 1. Average CHO expenditure after melatonin (M) ingestion for the total work period as well as for stages 2–5 were significantly (p < 0.05) greater than after the placebo (P) ingestion. There was no significant difference in CHO expenditure between M and P during stage 1.
Figure 1.
The average CHO expenditure for the entire 30 min of work as well as for Naughton Stages 1–5 after either placebo or melatonin ingestion. * indicates a CHO expenditure following melatonin ingestion that is significantly greater (p < 0.05) than following placebo ingestion.
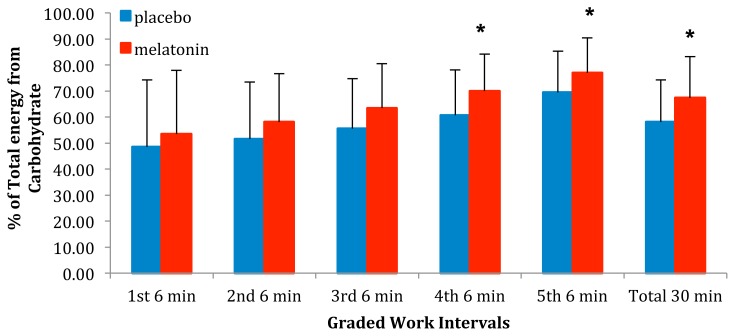
Figure 2 shows the relative contribution CHO made of the total kJ consumed. The relative contribution CHO made of the total kJ consumed for the entire 30 min of work as well as for stages 3–5 after melatonin (M) ingestion were significantly (p < 0.05) greater than after the placebo (P). There was no significant difference between M and P during Stages 1 & 2.
Figure 2.
The relative contribution from CHO to the total kJ consumed for the entire 30 min of work as well as for Naughton Stages 1–5 after either placebo or melatonin ingestion. * indicates a relative contribution from CHO following melatonin ingestion that is significantly greater (p < 0.05) than following placebo ingestion.
DISCUSSION
As mentioned above, notwithstanding the promotion of exogenous ingestion of melatonin as a treatment for metabolic syndrome (8, 20, 39), research data has not been completely supportive. For example, resting human studies have been contraindicative, with some researchers showing a relationship between melatonin and low blood glucose (13, 15), and others showing melatonin to be related to higher blood glucose levels (6, 31, 34). With respect to exercise, changes in human exercise substrate utilization with exogenous melatonin, however, are few with only Sanders et al. (36) reporting that blood glucose levels during graded exercise were higher following glucose ingestion. Therefore, the intent of this study was to supply initial information concerning the effect of melatonin on substrate utilization during graded exercise. This study showed that when physically active adults ingested melatonin 30 min prior to aerobic exercise, the predominant reliance upon CHO (Figure 2) to fuel the exercise occurred at a lower exercise intensity than following placebo ingestion. This earlier switch to CHO resulted in an increase in the total amount of CHO used during the exercise (Figure 1). Thus, it would appear that melatonin ingestion prior to exercise could enhance the anti-metabolic syndrome actions of exercise by decreasing any overabundance of blood glucose via increased usage.
While an increase in the removal and usage of blood glucose is desirable, before it can be conclusively stated that the coupling of melatonin ingestion with exercise would benefit individuals with metabolic syndrome, one must determine why melatonin increased CHO usage. It is well documented that increased blood glucose levels during exercise leads to enhanced glucose usage (7), and it is likely that the increased use of CHO is due a rise in blood glucose levels triggered by the ingestion of melatonin. As mentioned above, resting human studies have shown melatonin to be related to higher blood glucose levels (6, 31, 34). Moreover, long-term oral melatonin led to elevated plasma glucagon in Wistar rats, and this effect appeared to be greater when the rats were hyperglycemic (5). Additionally, Cagnacci et al. (6) showed that the raised blood glucose post-melatonin ingestion persisted for at least 180 min. Thus, it would appear that the increased use of glucose shown in this study was due a melatonin-induced elevation of blood glucose concentration.
In summary, the results of this study show that when melatonin is ingested by young active healthy individuals prior to exercise, there is an increased use of glucose as a fuel for the exercise. Unfortunately, the melatonin-induced increased use of glucose cannot ensure that a melatonin and exercise program will result in lower blood glucose post exercise. This is because the increased use of glucose was probably due to a melatonin-influenced rise in overall blood glucose levels. Therefore, caution should be placed upon the increased use of CHO during exercise as an example of melatonin benefiting individuals with metabolic syndrome. Rather this finding may point to a negative relationship between melatonin ingestion and metabolic syndrome. If the increased use of CHO is due to a melatonin induced increase in blood glucose, then it would be advisable to be engaged in exercise as soon as possible after the ingestion of the melatonin. Especially if that person is using melatonin to combat metabolic syndrome. Considering that our investigation only included 20–30 year old participants, future research could investigate those of middle age, poor fitness levels or those with Type 2 Diabetes. Since high melatonin encourages greater blood glucose use, it may be possible for those with Type 2 Diabetes to have a different response to exercise to aid in lowering blood glucose if they were to exercise in the morning as opposed to the evening/night. Additionally, future research investigating melatonin and blood glucose levels could incorporate individuals such as shift workers or patients with sleep disorders/sleep deprivation. Utilizing those types of participants may help to provide some insight on potential mechanisms responsible for the metabolic dysregulation within these populations.
REFERENCES
- 1.Acuna-Castroviejo D, Escames G, Rodriguez MI, Lopez LC. Melatonin role in the mitochondrial function. Front Biosci. 2007;12:947–963. doi: 10.2741/2116. [DOI] [PubMed] [Google Scholar]
- 2.Agil A, Rosado I, Ruiz R, Figueroa A, Zen N, Fernández-Vázquez G. Melatonin improves glucose homeostasis in young Zucker diabetic fatty rats. J Pineal Res. 2012;52(2):203–210. doi: 10.1111/j.1600-079X.2011.00928.x. [DOI] [PubMed] [Google Scholar]
- 3.Aoyama H, Mori N, Mori W. Effects of melatonin on genetic hypercholesterolemia in rats. Atherosclerosis. 1988;69(2–3):269–272. doi: 10.1016/0021-9150(88)90023-8. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson G, Drust B, Reilly T, Waterhouse J. The relevance of melatonin to sports medicine and science. Sports Med. 2003;33(11):809–831. doi: 10.2165/00007256-200333110-00003. [DOI] [PubMed] [Google Scholar]
- 5.Bähr I, Mühlbauer E, Schucht H, Peschke E. Melatonin stimulates glucagon secretion in vitro and in vivo. J Pineal Res. 2011;50(3):336–344. doi: 10.1111/j.1600-079X.2010.00848.x. [DOI] [PubMed] [Google Scholar]
- 6.Cagnacci A, Arangino S, Renzi A, Paoletti AM, Melis GB, Cagnacci P, Volpe A. Influence of melatonin administration on glucose tolerance and insulin sensitivity of postmenopausal women. Clin Endocrinol (Oxf) 2001;54(3):339–346. doi: 10.1046/j.1365-2265.2001.01232.x. [DOI] [PubMed] [Google Scholar]
- 7.Cermak NM, van Loon LJ. The use of carbohydrates during exercise as an ergogenic aid. Sports Med. 2013;43(11):1139–1155. doi: 10.1007/s40279-013-0079-0. [DOI] [PubMed] [Google Scholar]
- 8.Cipolla-Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ. Melatonin, energy metabolism, and obesity: a review. J Pineal Res. 2014;56(4):371–381. doi: 10.1111/jpi.12137. [DOI] [PubMed] [Google Scholar]
- 9.Cuzzocrea S, Reiter RJ. Pharmacological actions of melatonin in acute and chronic inflammation. Curr Top Med Chem. 2002;2(2):153–165. doi: 10.2174/1568026023394425. [DOI] [PubMed] [Google Scholar]
- 10.Daskalopoulou SS, Mikhailidis DP, Elisaf M. Prevention and treatment of the metabolic syndrome. Angiology. 2004;55(6):589–612. doi: 10.1177/00033197040550i601. [DOI] [PubMed] [Google Scholar]
- 11.Di Bella L, Gualano L. Key aspects of melatonin physiology: thirty years of research. Neuro Endocrinol Lett. 2006;27(4):425–432. [PubMed] [Google Scholar]
- 12.Gaesser GA. Exercise for prevention and treatment of cardiovascular disease, type 2 diabetes, and metabolic syndrome. Curr Diab Rep. 2007;7(1):14–19. doi: 10.1007/s11892-007-0004-8. [DOI] [PubMed] [Google Scholar]
- 13.Garfinkel D, Zorin M, Wainstein J, Matas Z, Laudon M, Zisapel N. Efficacy and safety of prolonged-release melatonin in insomnia patients with diabetes: a randomized, double-blind, crossover study. Diabetes Metab Syndr Obes. 2011;4:307–313. doi: 10.2147/DMSO.S23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibaldi M, Perrier D. Pharmacokinetics. New York: Dekker; 1982. [Google Scholar]
- 15.Hussain SA, Khadim HM, Khalaf BH, Ismail SH, Hussein KI, Sahib AS. Effects of melatonin and zinc on glycemic control in type 2 diabetic patients poorly controlled with metformin. Saudi Med J. 2006;27(10):1483–1488. [PubMed] [Google Scholar]
- 16.Johe PD, Osterud B. The in vivo effect of melatonin on cellular activation processes in human blood during strenuous physical exercise. J Pineal Res. 2005;39(3):324–330. doi: 10.1111/j.1600-079X.2005.00254.x. [DOI] [PubMed] [Google Scholar]
- 17.John TM, Viswanathan M, George JC, Scanes CG. Influence of chronic melatonin implantation on circulating levels of catecholamines, growth hormone, thyroid hormones, glucose, and free fatty acids in the pigeon. Gen Comp Endocrinol. 1990;79(2):226–232. doi: 10.1016/0016-6480(90)90107-w. [DOI] [PubMed] [Google Scholar]
- 18.Kaya O, Gokdemir K, Kilic M, Baltaci AK. Melatonin supplementation to rats subjected to acute swimming exercise: Its effect on plasma lactate levels and relation with zinc. Neuro Endocrinol Lett. 2006;27(1–2):263–266. [PubMed] [Google Scholar]
- 19.Kim E, Park H, Cha YS. Exercise training and supplementation with carnitine and antioxidants increases carnitine stores, triglyceride utilization, and endurance in exercising rats. J Nutr Sci Vitaminol (Tokyo) 2004;50(5):335–343. doi: 10.3177/jnsv.50.335. [DOI] [PubMed] [Google Scholar]
- 20.Korkmaz A, Topal T, Tan DX, Reiter RJ. Role of melatonin in metabolic regulation. Rev Endocr Metab Disord. 2009;10(4):261–270. doi: 10.1007/s11154-009-9117-5. [DOI] [PubMed] [Google Scholar]
- 21.Lakka TA, Laaksonen DE. Physical activity in prevention and treatment of the metabolic syndrome. Appl Physiol Nutr Metab. 2007;32(1):76–88. doi: 10.1139/h06-113. [DOI] [PubMed] [Google Scholar]
- 22.Lardone PJ, Alvarez-Sanchez SN, Guerrero JM, Carrillo-Vico A. Melatonin and glucose metabolism: clinical relevance. Curr Pharm Des. 2014;20(30):4841–4853. doi: 10.2174/1381612819666131119101032. [DOI] [PubMed] [Google Scholar]
- 23.Lusk G. Animal calorimetry. Twenty-fourth paper. Analysis of the oxidation of mixtures of carbohydrate and fat. A correction. J Biol Chem. 1924;59(1):41–42. [Google Scholar]
- 24.Maury E, Ramsey KM, Bass J. Circadian rhythms and metabolic syndrome: from experimental genetics to human disease. Circ Res. 2010;106(3):447–462. doi: 10.1161/CIRCRESAHA.109.208355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazepa RC, Cuevas MJ, Collado PS, González-Gallego J. Melatonin increases muscle and liver glycogen content in nonexercised and exercised rats. Life Sci. 2000;66(2):153–160. doi: 10.1016/s0024-3205(99)00573-1. [DOI] [PubMed] [Google Scholar]
- 26.Meeking DR, Wallace JD, Cuneo RC, Forsling M, Russell-Jones DL. Exercise-induced GH secretion is enhanced by the oral ingestion of melatonin in healthy adult male subjects. Eur J Endocrinol. 1999;141(1):22–26. doi: 10.1530/eje.0.1410022. [DOI] [PubMed] [Google Scholar]
- 27.Misigoj-Durakovic M, Durakovic Z. The early prevention of metabolic syndrome by physical exercise. Coll Antropol. 2009;33(3):759–764. [PubMed] [Google Scholar]
- 28.Mori N, Aoyama H, Murase T, Mori W. Anti-hypercholesterolemic effect of melatonin in rats. Acta Pathol Jpn. 1989;39(10):613–618. doi: 10.1111/j.1440-1827.1989.tb02407.x. [DOI] [PubMed] [Google Scholar]
- 29.Nassar E, Mulligan C, Taylor L, Kerksick C, Galbreath M, Greenwood M, Kreider R, Willoughby DS. Effects of a single dose of N-Acetyl-5-methoxytryptamine (Melatonin) and resistance exercise on the growth hormone/IGF-1 axis in young males and females. J Int Soc Sports Nutr. 2007;4:14. doi: 10.1186/1550-2783-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naughton JP. Methods of exercise testing. In: Naughton JP, Hellerstein HK, editors. Exercise testing and exercise training in coronary heart disease. New York: Academic; 1973. [Google Scholar]
- 31.Nelson AG. Dim light exposure reduces a type 2 diabetic’s glucoregulatory ability: A case study. J Gen Intern Med. 2012;27(Sup 2):560. [Google Scholar]
- 32.Nicolson GL. Metabolic syndrome and mitochondrial function: molecular replacement and antioxidant supplements to prevent membrane peroxidation and restore mitochondrial function. J Cell Biochem. 2007;100(6):1352–1369. doi: 10.1002/jcb.21247. [DOI] [PubMed] [Google Scholar]
- 33.Peschke E, Frese T, Chankiewitz E, Peschke D, Preiss U, Schneyer U, Spessert R, Mühlbauer E. Diabetic Goto Kakizaki rats as well as type 2 diabetic patients show a decreased diurnal serum melatonin level and an increased pancreatic melatonin-receptor status. J Pineal Res. 2006;40(2):135–143. doi: 10.1111/j.1600-079X.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 34.Radziuk J, Pye S. Diurnal rhythm in endogenous glucose production is a major contributor to fasting hyperglycaemia in type 2 diabetes. Suprachiasmatic deficit or limit cycle behaviour? Diabetologia. 2006;49(7):1619–1628. doi: 10.1007/s00125-006-0273-9. [DOI] [PubMed] [Google Scholar]
- 35.Sánchez-Campos S, Arévalo M, Mesonero MJ, Esteller A, González-Gallego J, Collado PS. Effects of melatonin on fuel utilization in exercised rats: role of nitric oxide and growth hormone. J Pineal Res. 2001;31(2):159–166. doi: 10.1034/j.1600-079x.2001.310210.x. [DOI] [PubMed] [Google Scholar]
- 36.Sanders DC, Bartschi TM, Trionfante CP, Kokkonen J, Nelson AG. A pre-exercise dose of melatonin can alter blood glucose levels during exercise. Med Sci Sports Exerc. 2015;47(Sup 1–5S):452. [Google Scholar]
- 37.Sartori C, Dessen P, Mathieu C, Monney A, Bloch J, Nicod P, Scherrer U, Duplain H. Melatonin improves glucose homeostasis and endothelial vascular function in high-fat diet-fed insulin-resistant mice. Endocrinology. 2009;150(12):5311–5317. doi: 10.1210/en.2009-0425. [DOI] [PubMed] [Google Scholar]
- 38.Srinivasan V. Melatonin oxidative stress and neurodegenerative diseases. Indian J Exp Biol. 2002;40(6):668–679. [PubMed] [Google Scholar]
- 39.Srinivasan V, Ohta Y, Espino J, Pariente JA, Rodriguez AB, Mohamed M, Zakaria R. Metabolic syndrome, its pathophysiology and the role of melatonin. Recent Pat Endocr Metab Immune Drug Discov. 2013;7(1):11–25. [PubMed] [Google Scholar]
- 40.Tengattini S, Reiter RJ, Tan DX, Terron MP, Rodella LF, Rezzani R. Cardiovascular diseases: protective effects of melatonin. J Pineal Res. 2008;44(1):16–25. doi: 10.1111/j.1600-079X.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 41.Zeman M, Výboh P, Juráni M, Lamosová D, Kostal L, Bilcík B, Blazícek P, Jurániová E. Effects of exogenous melatonin on some endocrine, behavioural and metabolic parameters in Japanese quail Coturnix coturnix japonica. Comp Biochem Physiol Comp Physiol. 1993;105(2):323–328. doi: 10.1016/0300-9629(93)90215-p. [DOI] [PubMed] [Google Scholar]