Abstract
The bone marrow microenvironment is known to provide a survival advantage to residual acute myeloid leukemia cells, possibly contributing to disease recurrence. The mechanisms by which stroma in the microenvironment regulates leukemia survival remain largely unknown. Using reverse-phase protein array technology, we profiled 53 key protein molecules in 11 signaling pathways in 20 primary acute myeloid leukemia samples and two cell lines, aiming to understand stroma-mediated signaling modulation in response to the targeted agents temsirolimus (MTOR), ABT737 (BCL2/BCL-XL), and Nutlin-3a (MDM2), and to identify the effective combination therapy targeting acute myeloid leukemia in the context of the leukemia microenvironment. Stroma reprogrammed signaling networks and modified the sensitivity of acute myeloid leukemia samples to all three targeted inhibitors. Stroma activated AKT at Ser473 in the majority of samples treated with single-agent ABT737 or Nutlin-3a. This survival mechanism was partially abrogated by concomitant treatment with temsirolimus plus ABT737 or Nutlin-3a. Mapping the signaling networks revealed that combinations of two inhibitors increased the number of affected proteins in the targeted pathways and in multiple parallel signaling, translating into facilitated cell death. These results demonstrated that a mechanism-based selection of combined inhibitors can be used to guide clinical drug selection and tailor treatment regimens to eliminate microenvironment-mediated resistance in acute myeloid leukemia.
Introduction
Acute myeloid leukemia (AML) has a high initial treatment response rate, associated with the elimination of bulk leukemic cells, and an almost inevitable high relapse rate.1,2 Recent studies indicate that stroma in the bone marrow (BM) microenvironment protects resident leukemic cells and plays a key role in AML relapse.3–7 Activation of the PI3K/AKT/MTOR pathway, upregulation of the anti-apoptotic BCL2 family and MDM2/P53 signaling have been identified in patients with disease recurrence8–13 and associated with stroma-mediated AML survival.14–18 Strategies for targeting the key molecules in these pathways have been developed to improve therapeutic efficacy in patients with AML.19
Temsirolimus, ABT737, and Nutlin-3a are selective small-molecule inhibitors that affect MTOR, BCL2/BCL-XL and MDM2/P53 signaling, respectively. Temsirolimus, a rapamycin analog and cytostatic inhibitor, prevents leukemic cell proliferation by blocking the formation of MTOR complex 1 (MTORC1) and MTOR complex 2 (MTORC2) and sequentially inactivating AKT/MTOR downstream signaling.20,21 ABT737, a selective small-molecule BCL2/BCL-XL antagonist, exerts its proapoptotic function by preventing BCL2 family proteins from sequestering to activate BH3-only proteins.22,23 Nutlin-3a, a small-molecule MDM2 inhibitor, binds to MDM2 in the P53-binding pocket and activates P53-mediated apoptosis.24,25
The efficacy of these inhibitors, both as single agents and in combination, has been evaluated in preclinical studies of hematological malignancy.23,26–29 Although high potency was reported in these studies, only a modest therapeutic response was observed in clinical trials.30–32 This inconsistency between preclinical results and clinical outcomes is attributable to two factors. First, most of the preclinical studies were performed under in vitro monolayer conditions that did not account for the possible influence of the microenvironment on the effectiveness of the targeted inhibitors. Second, the on-target effects of temsirolimus, ABT737, and Nutlin-3a were frequently examined only for their target-specific pathways PI3K/AKT/MTOR, BCL2/BCL-XL, and MDM2/P53 without considering parallel signaling. This focus precluded assessment of survival mechanisms mediated by compensatory signaling networks. Thus, the microenvironment-modulated signaling networks of single and combined targeted inhibitors require further investigation. Results of such studies will contribute to the development of effective treatments to target microenvironment-mediated AML survival.
Reverse-phase protein array (RPPA), a high-throughput functional proteomic technology, facilitates broad and simultaneous profiling of therapeutically relevant signaling networks. This technique has been successfully used to identify signaling pathway abnormalities, pharmacodynamic markers, and proteins associated with therapeutic resistance in various cancers, including leukemia.33 In the study herein, using RPPA technology, we profiled 53 key molecules in 11 signaling pathways in 20 primary AML samples and two AML cell lines. Our goals were to understand the role of microenvironment-mediated signaling in AML survival by comparing the signaling network alterations triggered by temsirolimus, ABT737, and Nutlin-3a in samples cultured alone and co-cultured with stroma, a condition mimicking the BM microenvironment, and to identify effective combination strategies targeting stroma-regulated AML. Our results indicate that stroma-mediated signaling is specific to each targeted inhibitor. By mapping the network alterations triggered by the combination of temsirolimus plus ABT737 or Nutlin-3a, we revealed the mechanisms by which combinatorial treatment abrogated stroma-mediated survival and facilitated leukemic cell death. Our findings provide a clinically relevant approach for selecting mechanism-based therapy to effectively eliminate microenvironment-protected AML.
Methods
Materials, cell lines, and patient samples
Information about the materials and cell lines used in this study is provided in the Online Supplementary Materials and Methods. Clinical information about the primary AML samples is provided in the Online Supplementary Table S1. All samples were collected during routine diagnostic procedures in accordance with protocols approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center. Informed consent was obtained in accordance with the Declaration of Helsinki.
Co-culture of leukemic cells and stromal cells
MS-5 is an established murine stromal cell line that has been routinely used for long-term support of primary hematopoietic cells and demonstrated high stability.14,34 Unlike human BM-derived mesenchymal stromal cells (MSCs), the function of MS-5 is less affected by cell passages. We chose MS-5 to construct a model of the BM microenvironment for the RPPA experiment and used normal human MSCs to verify RPPA analyses.
Stromal cells were plated in 10% fetal bovine serum (FBS) α-minimum essential medium and cultured overnight. The culture medium was then removed, and leukemic cells were seeded on top of stromal cells at a ratio of 10:1 (leukemic:stromal cells) in 10% FBS Roswell Park Memorial Institute (RPMI) 1640 medium. Leukemic and stromal cells were co-cultured for three hours before being exposed to single or combined agents at the concentrations specified below.
Cell treatment
Leukemic cells alone or in co-culture with stromal cells were treated with the following concentrations of a single agent or a combination of two agents: temsirolimus (2.42 μM),21 ABT737 (50 nM, except for OCI-AML3 cells treated at 0.25 μM in RPPA, 0.1 or 0.25 μM in immunoblotting),23 and Nutlin-3a (5 μM).25 RPPA and immunoblotting analyses were performed on cells that had been treated for 24 hours; apoptosis induction was measured on cells treated for 72 hours.
Apoptosis assay
Cell apoptosis was analyzed by flow cytometry of annexin V (Roche Diagnostics, Indianapolis, IN, USA) and propidium iodide (PI) (Sigma Chemical, St. Louis, MO, USA) positivity on a gated CD45+ population (anti-human CD45, BD Pharmingen, San Diego, CA, USA). To examine and compare inhibitor-specific apoptosis, we calculated % of specific apoptosis as previously described: % specific apoptosis = (tested − control) / (100 − control) × 100, where “tested” is the percentage of annexin V/PI-positivity in treated cells, and “control” is the percentage of annexin V/PI-positivity in untreated cells (spontaneous apoptosis).25
Immunoblotting
Protein expression in treated and untreated cells was determined by immunoblotting. Protein signals were detected using an Odyssey Infrared Imaging System and quantified using Odyssey software version 3.0 (LI-COR Biosciences, Lincoln, NE, USA).
RPPA
RPPA was performed on two AML cell lines, OCI-AML3 and U937, and 20 primary AML samples with high blast counts (Online Supplementary Table S1). Cells cultured alone or co-cultured with MS-5 were harvested after 24 hours of treatment. Co-cultured leukemic cells were collected according to a previously published protocol.35 To exclude the possibility of the leukemic cells being contaminated by MS-5, flow cytometry was used to verify the absence of a CD90+ (anti-mouse CD90, BD Pharmingen) population among the leukemic cells. The collected cells were then lysed and subjected to RPPA using previously described and validated methods.33,36 Raw signal intensities obtained from RPPA were processed with SuperCurve to determine relative protein concentrations, and the results were further normalized to adjust for loading bias by median-centering each marker and each sample.37,38
Statistical analysis
We used a two-sided “fold-change-filtered” binomial test and a two-tailed Student’s t-test to identify the distinct protein and pathway alterations and apoptosis induction triggered by treatment and mediated by stroma. The detailed statistical analyses are described in the Online Supplementary Materials and Methods.
Results
RPPA profiling of key molecules in signaling networks
To investigate treatment-mediated signaling networks, we profiled 53 key proteins in 11 signaling pathways: (1) PI3K/AKT/MTOR signaling, (2) AKT/MTOR major downstream signaling, (3) MEK/ERK signaling, (4) STAT3 signaling, (5) BCL2 protein family, (6) WNT CATENIN signaling, (7) P53 family, (8) IAP family, (9) cell cycle regulation, (10) PP2A phosphatase family, and (11) MYC signaling (Online Supplementary Figure S1; Online Supplementary Table S2), in two AML cell lines, OCI-AML3 and U937, and 20 primary AML samples. Treatment-induced apoptosis with or without stroma was measured for 15 of the 20 primary samples.
Stroma altered target proteins and the sensitivity of AML to temsirolimus
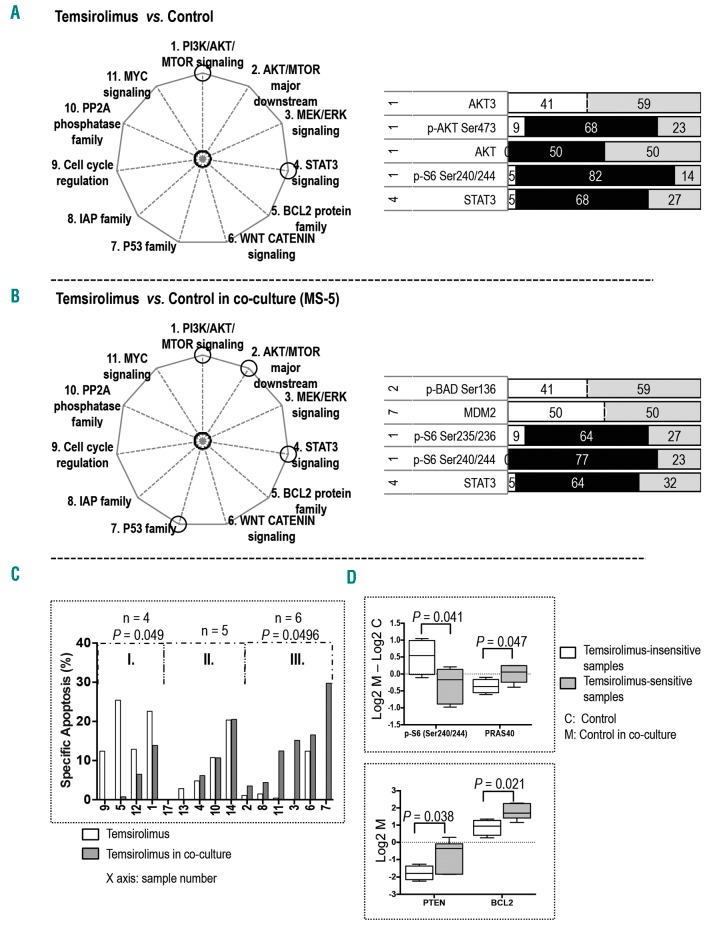
In samples cultured alone, the MTOR inhibitor temsirolimus significantly affected five proteins in two signaling pathways; four in the PI3K/AKT/MTOR signaling and one in the STAT3 pathway (Figure 1A; Online Supplementary Figure S2A). Temsirolimus treatment inhibited p-AKT (Ser473) and p-S6 (Ser240/244), upregulated AKT3, and decreased the expression of total AKT and STAT3.
Figure 1.
Stroma altered target proteins and the sensitivity of AML to temsirolimus. The pathways affected by temsirolimus treatment are circled in the pie charts on the left. The bar graphs display proteins whose expression was significantly altered in (A) monocultured samples treated with temsirolimus versus untreated control (n = 22) and (B) co-cultured samples treated with temsirolimus versus untreated control (n = 22). The numbers on the left are the numbers of the pathways (as indicated on the pie chart and in the Online Supplementary Figure S1). The black area indicates the percentage of affected AML samples ([number of affected samples / total number of samples] × 100) that exhibited downregulation of protein expression. The white area indicates the percentage of samples with upregulation of protein expression. The gray area represents the percentage of samples having no significant changes in protein expression. (C) Bar graph displays temsirolimus-induced specific apoptosis of samples cultured alone and co-cultured with stroma. The sample groupings and the statistical calculation of apoptosis for each defined group are described in the Online Supplementary Materials and Methods. (D) Box and whisker plots display differences in protein expression between untreated samples in groups I and III with and without stroma (top panel) and protein expression at the baseline level of untreated samples in groups I and III in co-culture (bottom panel). Whiskers indicate the range from minimum to maximum values. The line in the middle of the box is plotted at the median.
In co-cultured samples, temsirolimus treatment affected five proteins in four pathways: two in the PI3K/AKT/MTOR pathway, one in the AKT/MTOR major downstream signaling, one in the P53 family, and one in the STAT3 signaling (Figure 1B; Online Supplementary Figure S2B). Temsirolimus treatment inhibited p-S6 at Ser235/236 and Ser240/244 in the PI3K/AKT/MTOR pathway, upregulated p-BAD (Ser136) in the AKT/MTOR major downstream signaling, and suppressed STAT3 expression. Interestingly, under stromal co-culture, temsirolimus treatment triggered upregulation of MDM2, a ubiquitin ligase responsible for P53 degradation.
In stromal co-culture, temsirolimus-induced apoptosis was decreased in four samples, increased in six samples, and unchanged in five samples (Figure 1C). RPPA analysis of untreated samples showed that the stroma-mediated upregulation of p-S6 (Ser240/244) and downregulation of PRAS40 was significantly greater in temsirolimus-insensitive samples than in temsirolimus-sensitive samples; in addition, temsirolimus-insensitive samples had a lower baseline expression of PTEN and BCL2 than did the temsirolimus-sensitive samples in co-culture (Figure 1D). Together, these results suggest that modulation of the sensitivity of AML cells to temsirolimus was the result of stroma-regulated alterations in signaling networks.
Stroma altered target proteins and the sensitivity of AML to ABT737
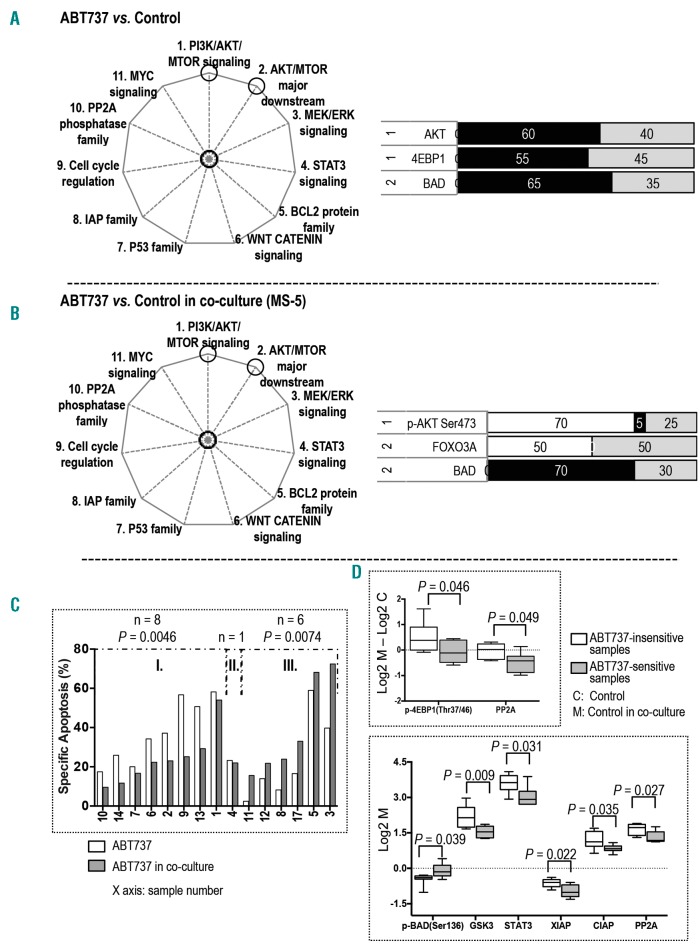
In samples cultured alone, treatment with the BCL2/BCL-XL inhibitor ABT737 significantly affected three proteins in two pathways; two in the PI3K/AKT/MTOR pathway and one in the AKT/MTOR major downstream signaling (Figure 2A; Online Supplementary Figure S3A). ABT737 treatment suppressed the expression of AKT and 4EBP1 in the PI3K/AKT/MTOR pathway and decreased BAD expression in the AKT/MTOR major downstream signaling.
Figure 2.
Stroma altered target proteins and the sensitivity of AML to ABT737. The pathways affected by ABT737 treatment are circled in the pie charts on the left. The bar graphs display proteins whose expression was significantly altered in (A) monocultured samples treated with ABT737 versus untreated control (n = 20) and (B) co-cultured samples treated with ABT737 versus untreated control (n = 20). The numbers on the left are the numbers of the pathways (as indicated on the pie chart and in the Online Supplementary Figure S1). The calculation of the black, white, and gray area and the color key on the bar graphs are described in the legend for Figure 1. (C) Bar graph displays ABT737-induced specific apoptosis for samples cultured alone and co-cultured with stroma. The sample groupings and the statistical calculation of apoptosis are described in the Online Supplementary Materials and Methods. (D) Box and whisker plots display differences in protein expression between untreated samples in groups I and III with and without stroma (top panel) and protein expression at the baseline level of untreated samples in groups I and III in co-culture (bottom panel). Whiskers indicate the range from minimum to maximum values. The line in the middle of the box is plotted at the median.
In stromal co-culture, ABT737 treatment significantly affected three proteins in two pathways; one in the PI3K/AKT/MTOR pathway and two in the AKT/MTOR major downstream signaling (Figure 2B; Online Supplementary Figure S3B). ABT737 treatment, contrary to its effects without stroma, upregulated p-AKT (Ser473) in the PI3K/AKT/MTOR pathway. It also increased the expression of FOXO3A and inhibited BAD in the AKT/MTOR major downstream signaling.
ABT737-induced apoptosis was decreased in eight and increased in six samples under co-culture (Figure 2C). RPPA analysis of untreated samples indicated that stroma-mediated upregulation of p-4EBP1 (Thr37/46) and PP2A was significantly greater in the eight insensitive samples than in the ABT737-sensitive samples; in addition, the insensitive samples had higher baseline expression of GSK3, STAT3, XIAP, CIAP, and PP2A and lower level of p-BAD (Ser136) than did the ABT737-sensitive samples in co-culture (Figure 2D). These results suggest that stroma reprogrammed signaling networks, thus altering the sensitivity of AML cells to ABT737.
Stroma altered target proteins and the sensitivity of AML to Nutlin-3a
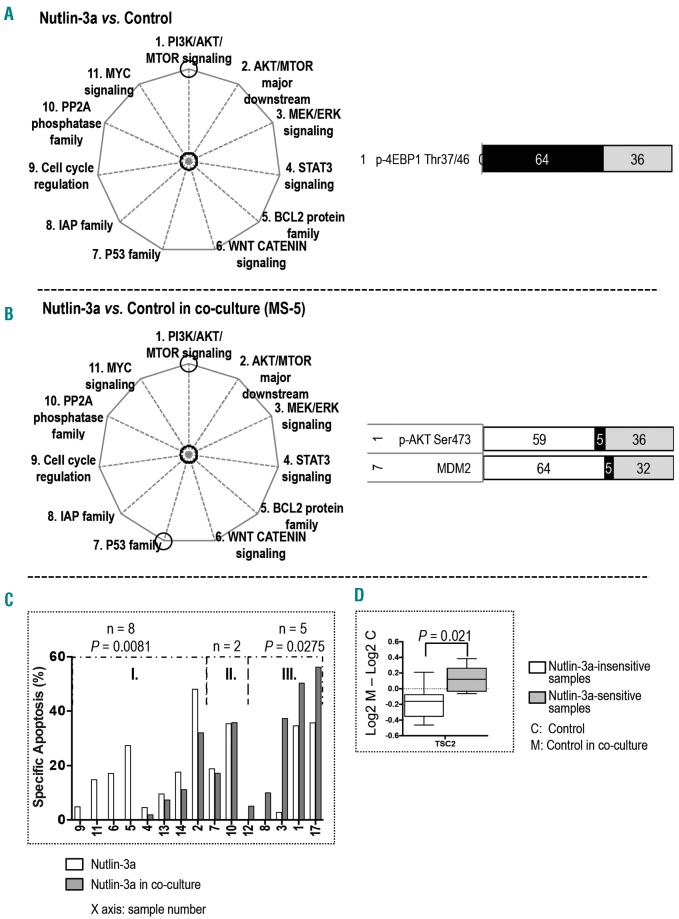
In samples cultured alone, treatment with the MDM2 inhibitor Nutlin-3a significantly inhibited p-4EBP1 (Thr37/46) in the PI3K/AKT/MTOR signaling (Figure 3A; Online Supplementary Figure S4A).
Figure 3.
Stroma altered target proteins and the sensitivity of AML to Nutlin-3a. The pathways affected by Nutlin-3a treatment are circled in the pie charts on the left. The bar graphs display proteins whose expression was significantly altered in (A) monocultured samples treated with Nutlin-3a versus untreated control (n = 22) and (B) co-cultured samples treated with Nutlin-3a versus untreated control (n = 22). The numbers on the left are the numbers of the pathways (as indicated on the pie chart and in the Online Supplementary Figure S1). The calculation of the black, white, and gray area and the color key on the bar graphs are described in the legend for Figure 1. (C) Bar graph displays Nutlin-3a-induced specific apoptosis for samples cultured alone and co-cultured with stroma. The sample groupings and the statistical calculation of apoptosis are described in the Online Supplementary Materials and Methods. (D) Box and whisker plots display differences in protein expression between untreated samples in groups I and III with and without stroma. Whiskers indicate the range from minimum to maximum values. The line in the middle of the box is plotted at the median.
In samples co-cultured with stroma, Nutlin-3a significantly affected two proteins in two signaling pathways; treatment upregulated p-AKT (Ser473) in the PI3K/AKT/MTOR signaling and increased MDM2 expression in the P53 family (Figure 3B; Online Supplementary Figure S4B).
Nutlin-3a-induced apoptosis was decreased in eight and increased in five samples under co-culture (Figure 3C). RPPA analysis of untreated samples demonstrated that the stroma-mediated downregulation of TSC2 was greater in the eight samples that were insensitive to Nutlin-3a than in the sensitive samples (Figure 3D). However, baseline protein expression between co-cultured Nutlin-3a-sensitive and insensitive samples were not significantly different.
Combined blockade of PI3K/AKT/MTOR and BCL2 signaling with temsirolimus and ABT737 demonstrated higher efficacy in AML under co-culture
Stroma-mediated AKT activation in samples treated with ABT737 indicates that PI3K/AKT/MTOR signaling is the survival mechanism triggered by BCL2/BCL-XL inhibition. This finding prompted us to evaluate the therapeutic effect of combined inhibition of PI3K/AKT/MTOR and BCL2 in AML samples co-cultured with stroma.
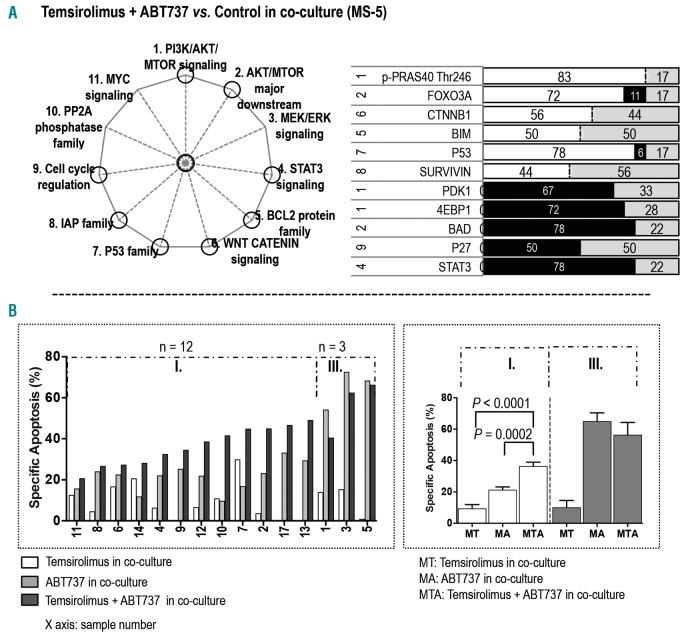
In co-culture, treatment with temsirolimus and ABT737 targeted more proteins than did treatment with temsirolimus or ABT737 alone. The two-inhibitor combination significantly affected 11 proteins in eight signaling pathways: three proteins in PI3K/AKT/MTOR signaling, two in the AKT/MTOR major downstream pathway, one in the IAP family, one in the BCL2 family, one in the P53 family, one in the WNT CATENIN pathway, one in cell cycle regulation, and one in STAT3 signaling (Figure 4A; Online Supplementary Figure S5A). In the PI3K/AKT/MTOR pathway, combination treatment suppressed the expression of PDK1 and 4EBP1 and upregulated p-PRAS40 (Thr246). In the AKT/MTOR major downstream signaling, this combination upregulated FOXO3A and inhibited BAD expression. ABT737 plus temsirolimus decreased the expression of STAT3 and cell cycle protein P27 and increased the expression of P53 and BCL2 family protein BIM. This co-treatment also upregulated CTNNB1 in the WNT CATENIN signaling and increased SURVIVIN expression in the IAP pathway.
Figure 4.
Combined blockade of PI3K/AKT/MTOR and BCL2 signaling with temsirolimus and ABT737 demonstrated higher efficacy in AML under co-culture. (A) The pathways affected by combination treatment with temsirolimus and ABT737 are circled in the pie chart on the left. The bar graph displays proteins whose expression was significantly altered in samples treated with the combination of temsirolimus and ABT737 (n = 18) versus the matched samples without treatment in co-culture. The numbers on the left are the numbers of the pathways (as indicated on the pie chart and in the Online Supplementary Figure S1). The calculation of the black, white, and gray area and the color key on the bar graph are described in the legend for Figure 1. (B) The bar graph on the left displays the specific apoptosis for each co-cultured sample treated with temsirolimus, ABT737, and the combination of temsirolimus and ABT737. The sample groupings and the statistical calculation of apoptosis are described in the Online Supplementary Materials and Methods. The bar graph on the right displays the specific apoptosis in each group. Values are presented as mean ± standard error of the mean.
The combination treatment significantly restored drug sensitivity and facilitated cell death in 12 of 15 co-cultured samples (Figure 4B). Among the 12 samples, combination increased the sensitivity of three samples that were resistant to temsirolimus (samples 9, 13, 17) and two samples that were insensitive to ABT737 (10, 14). Mechanistically, the co-treatment repressed the ABT737-upregulated p-AKT (Ser473) in four samples (4, 7, 8, 11) and decreased p-AKT expression that had been unaffected by ABT737 in two samples (2, 9). The combination did not further enhance apoptosis in three samples (1, 3, 5) that had various levels of ABT737-induced p-AKT expression and were highly sensitive to ABT737 single-agent treatment (Online Supplementary Figures S3B and S5B).
Taken together, these results indicate that co-inhibition of PI3K/AKT/MTOR and BCL2/BCL-XL overcomes stroma-mediated AML survival, resulting in increased therapeutic efficacy against most AMLs examined.
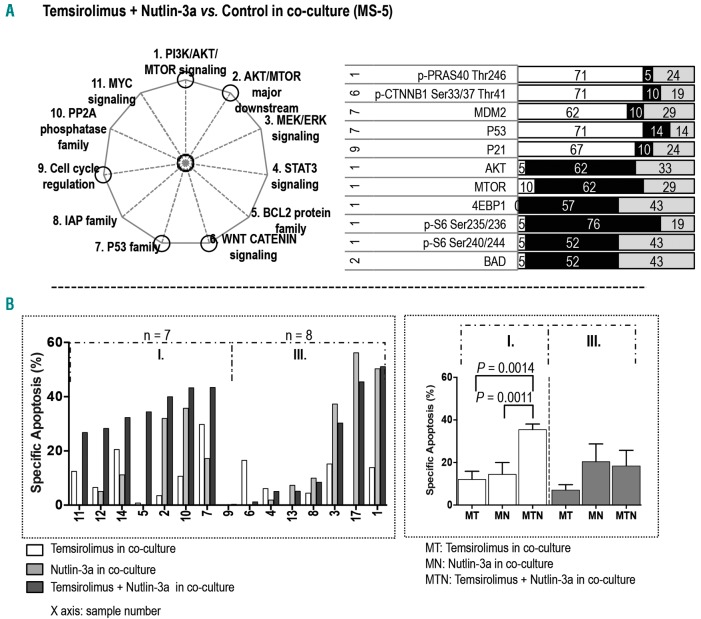
Combined blockade of PI3K/AKT/MTOR and MDM2 signaling with temsirolimus and Nutlin-3a demonstrated higher efficacy in AML under co-culture
Stroma-mediated AKT activation in samples treated with Nutlin-3a indicates that PI3K/AKT/MTOR signaling is the survival mechanism triggered by MDM2 inhibition. This finding prompted us to evaluate the therapeutic effect of combined inhibition of PI3K/AKT/MTOR and MDM2 in AML samples co-cultured with stroma.
Treatment with temsirolimus and Nutlin-3a affected 11 proteins in five pathways in co-cultured samples: six in the PI3K/AKT/MTOR signaling, one in the AKT/MTOR major downstream pathway, two in the P53 family, one in the WNT CATENIN pathway, and one in cell cycle regulation (Figure 5A; Online Supplementary Figure S6A). In the PI3K/AKT/MTOR pathway, the two-inhibitor combination downregulated p-S6 at Ser235/236 and Ser240/244, decreased the expression of AKT, MTOR, and 4EBP1, and upregulated p-PRAS40 (Thr246). This combination also decreased BAD expression in the AKT/MTOR major downstream pathway and upregulated the cell cycle protein P21. Furthermore, it increased the expression of MDM2 and P53 in the P53 family. Finally, this co-treatment resulted in upregulation of p-CTNNB1 (Ser33/37/Thr41) in the WNT CATENIN signaling.
Figure 5.
Combined blockade of PI3K/AKT/MTOR and MDM2 signaling with temsirolimus and Nutlin-3a demonstrated higher efficacy in AML under co-culture. (A) The pathways affected by combination treatment with temsirolimus and Nutlin-3a are circled in the pie chart. The bar graph displays proteins whose expression was significantly altered in samples treated with the combination of temsirolimus and Nutlin-3a (n = 21) versus the matched samples without treatment in co-culture. The numbers on the left are the numbers of the pathways (as indicated on the pie chart on the left and in the Online Supplementary Figure S1). The calculation of the black, white, and gray area and the color key on the bar graph are described in the legend for Figure 1. (B) The bar graph on the left displays the specific apoptosis for each co-cultured sample treated with temsirolimus, Nutlin-3a, and the combination of temsirolimus and Nutlin-3a. The sample groupings and the statistical calculation of apoptosis are described in the Online Supplementary Materials and Methods. The bar graph on the right displays the specific apoptosis in each group. Values are presented as mean ± standard error of the mean.
The co-treatment of temsirolimus and Nutlin-3a significantly facilitated cell death in seven of 15 co-cultured samples (Figure 5B). It restored the sensitivity of three samples that were insensitive to temsirolimus (samples 2, 5, 12) and four samples that were either resistant (5, 11) or insensitive to Nutlin-3a (12, 14). Among these seven samples, p-AKT upregulation triggered by Nutlin-3a and inhibited by the co-treatment of temsirolimus and Nutlin-3a was observed in four samples (5, 7, 11, 12). The combination had no effect or a reduced effect in eight samples. Nutlin-3a-upregulated p-AKT was found in three of the eight samples (1, 8, 17), suggesting that PI3K/AKT/MTOR-independent survival signaling played a role in treatment sensitivity in the remaining five samples (Online Supplementary Figures S4B and S6B).
Together, our results suggest that combined inhibition of PI3K/AKT/MTOR and MDM2 with temsirolimus and Nutlin-3a can restore drug sensitivity in some co-cultured AML samples. However, the overall efficacy of this combination was inferior to that of co-inhibition of PI3K/AKT/MTOR and BCL2/BCL-XL with temsirolimus and ABT737.
RPPA validation
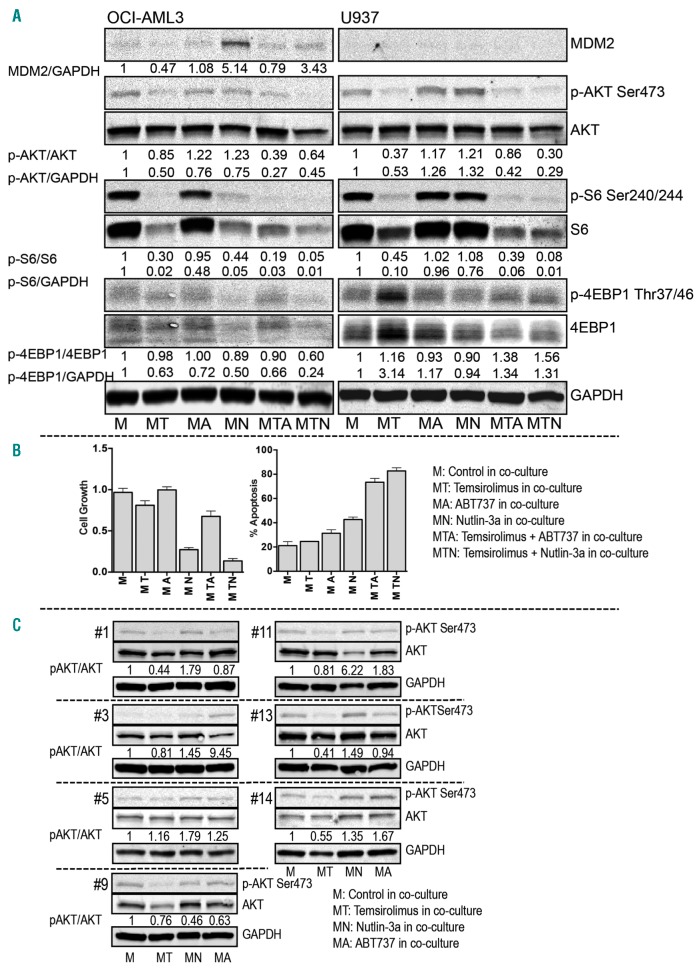
To validate the RPPA analysis, we performed separate experiments treating OCI-AML3 and U937 cells co-cultured with a healthy BM-derived MSC. Treated cells were examined via conventional immunoblotting (Figure 6A). Results demonstrated that temsirolimus inhibited p-AKT (Ser473) and p-S6 (Ser240/244) in co-cultured OCI-AML3 and U937 cells. Temsirolimus treatment also inhibited p-4EBP1 (Thr37/46) in OCI-AML3 cells but upregulated it in U937 cells. Nutlin-3a treatment resulted in MDM2 upregulation and p-S6 (Ser240/244) inhibition in OCI-AML3 cells, p-AKT (Ser473) upregulation in U937 cells and p-4EBP1 (Thr37/46) inhibition in both OCI-AML3 and U937 cells. ABT737 treatment inhibited p-S6 (Ser240/244) in OCI-AML3 cells and upregulated p-AKT (Ser473) in U937 cells. It modestly affected MDM2 in OCI-AML3, and 4EBP1 in both OCI-AML3 and U937 cells. Treatment with a combination of temsirolimus and ABT737 or Nutlin-3a partially repressed MDM2 upregulation in OCI-AML3, abrogated p-AKT upregulation triggered by ABT737 and Nutlin-3a in U937, and synergistically inhibited p-AKT (Ser473) and p-S6 (Ser240/244) in both cell lines. The immunoblotting findings that single-agent treatment induced up- or downregulation of p-AKT, p-S6, p-4EBP1, and MDM2 were generally consistent with the data obtained by RPPA in both cell lines co-cultured with MS-5 (Online Supplementary Figure S7A). Co-treatment enhanced cell death, and growth inhibition was confirmed in the co-cultured OCI-AML3 cells (Figure 6B). In addition, we conducted studies juxtaposing the effect of treatment on OCI-AML3 co-cultured with MS-5 and different healthy BM MSCs. The level of protection against treatment-induced cell death provided by various MSCs and MS-5 in OCI-AML3 cells was very similar (Online Supplementary Figure S7B). These results indicate that combination treatment with temsirolimus plus ABT737 or Nutlin-3a was more effective than single-agent treatment under conditions mimicking the BM microenvironment.
Figure 6.
Immunoblotting analysis validating RPPA results in AML cell lines and primary AML samples co-cultured with stroma. (A) Immunoblots of co-cultured OCI-AML3 and U937 cells treated with single inhibitor or dual inhibitors at the same concentration used in RPPA for 24 hours. The expression level of phosphorylated proteins (p-protein), total proteins, MDM2, and GAPDH was quantified. The ratios of the density of p-protein to that of total protein or GAPDH and of MDM2 to GAPDH were calculated and then divided by the density ratios of the same proteins in untreated cells. (B) Bar graphs display growth inhibition and apoptosis induction in co-cultured OCI-AML3 cells under the indicated treatments for 72 hours. Values are presented as mean ± standard deviation of the mean. (C) Lysates of primary AML samples analyzed using RPPA were reanalyzed using immunoblotting to detect the expression of p-AKT (Ser473) and AKT in the treated and untreated cells under co-culture. The bands were quantified, and the density ratios were calculated as described above.
To confirm the RPPA results in primary samples, we compared p-AKT (Ser473) detected via immunoblotting and RPPA in the same cell lysates harvested from primary samples treated with single-agent temsirolimus, ABT737, or Nutlin-3a in co-culture. Similar results of inhibitor-triggered p-AKT up- or downregulation were observed in all seven samples (Figure 6C and Online Supplementary Figure S7C).
Taken together, the overall similarities in the results obtained using RPPA and conventional immunoblotting confirmed the reliability of RPPA technology and provided a second line of evidence supporting the conclusions of the RPPA analysis.
AML heterogeneity and responses under stromal co-culture
Given the well-known heterogeneity of AML, we analyzed the responses to the targeted inhibitors and proteomic profiles with respect to disease status at the time of sampling (newly diagnosed vs. relapsed) and AML characteristics (molecular or cytogenetic subtypes). We found no statistically significant correlations with disease status or cytogenetics. However, four AML samples harboring FLT3 mutations were universally less sensitive to dual BCL2/BCL-XL inhibitor ABT737 and were instead responsive to the combination of temsirolimus plus ABT737 in stromal co-culture (Online Supplementary Figure S8A). RPPA analysis revealed stroma-dependent protein alterations in multiple pathways, including the PI3K/AKT/MTOR and BCL2 signaling, which could be selectively associated with FLT3-mutated AML (Online Supplementary Figure S8B).
Discussion
In this study, we used RPPA technology to examine cellular signaling alterations triggered by the blockade of MTOR, BCL2/BCL-XL, and MDM2 in AML cells under monoculture and stromal co-culture conditions. We identified the mechanisms by which stroma reprograms signaling networks in response to temsirolimus, ABT737, and Nutlin-3a and demonstrated that stroma-altered drug sensitivity is a result of signaling modulation. We further demonstrated that the PI3K/AKT/MTOR pathway is one of the major stroma-regulated survival pathways triggered by BCL2/BCL-XL and MDM2 inhibition. We identified the differences in the stroma-mediated networks affected by the combinations of temsirolimus plus ABT737 and temsirolimus plus Nutlin-3a and demonstrated that the simultaneous blockade of PI3K/AKT/MTOR and BCL2 is an effective strategy to combat stroma-mediated AML survival. These findings, which we confirmed via conventional immunoblotting, provide a clinically relevant and mechanism-based tool for selecting effective combination therapies to treat microenvironment-protected AML.
RPPA profiling of the signaling networks revealed that targeted inhibition affects proteins in non-targeted pathways. Our findings that STAT3 was inhibited by temsirolimus and that 4EBP1 expression was affected by Nutlin-3a are consistent with those of published reports;27,39 however, the finding that BAD expression was downregulated by ABT737 is documented here for the first time. Whether this effect is a result of blocking the downstream signaling cascade, of pathway crosstalk, or an off-target effect remains to be addressed in a future study.
Our results showing signaling network differences in AML with and without stroma confirm the physiological role of stroma in AML and support the need to study target-selective inhibitors in the context of the BM microenvironment. Our results provide key evidence that PI3K/AKT/MTOR signaling is a stroma-regulated survival mechanism for AML. We are the first to report that the stroma-mediated mutual response of AML to ABT737 and Nutlin-3a activates AKT. These findings suggest that signaling crosstalk between BCL2 and MDM2 may take place in the setting of stroma-leukemia interaction. Indeed, Matter and Ruoslahti40 reported that extracellular integrin α5β1-αVβ3-mediated BCL2 transcription occurs through the activation of AKT in the PI3K/AKT/MTOR signaling; similarly, Du et al.41 showed that insulin-like growth factor 1 (IGF-1) upregulates MDM2 expression through the PI3K/AKT/MTOR pathway. Thus, the direct blockade of BCL2 or MDM2 may trigger a feedback response that promotes the stroma-leukemia interaction via the surface molecules α5β1-αVβ3 or by enhancing stromal IGF-1 secretion, either of which would lead to AKT activation. Directly targeting the stroma-leukemia interaction to prevent these feedback responses might synergize the therapeutic effect of BCL2 and MDM2 inhibition. Such a therapeutic strategy is currently under investigation.
The synergistic effect of PI3K/AKT/MTOR and BCL2 blockade was demonstrated in a recent in vivo study in AML42 and in an in vitro study of solid tumor cells that formed spheroids, three-dimensional structures mimicking tumor cell growth in the microenvironment.43 Importantly, these findings provide a basis for investigating potential synergy at reduced doses, whereby combination treatment increases the therapeutic index without affecting overall therapeutic efficacy. Such an adjustment could be clinically valuable for reducing on-target toxicities associated with high doses of BCL2 or BCL-XL inhibitors, such as tumor lysis syndrome with ABT19932 and thrombocytopenia with ABT236.44
The combination of temsirolimus plus Nutlin-3a at 5 μM25 was less effective than the combination of temsirolimus plus ABT737. Recently, Lee et al.45 showed that the efficacy of combination treatment is affected by changes in the order and duration of drug exposure. Therefore, optimizing the administration sequence of temsirolimus and Nutlin-3a or the length of exposure to the single and combined treatments may increase target availability and improve therapeutic outcomes. Further studies should investigate these options. In addition, the toxicity and tolerance of this combination should be evaluated in an in vivo study.
Our findings demonstrated that the response of AML to targeted inhibition of PI3K/AKT/MTOR, BCL2/BCL-XL, and MDM2 is heterogeneous and complex and support the feasibility of personalized AML treatments. AMLs that are highly sensitive to ABT737 or Nutlin-3a alone may not require combination treatment. AMLs which are less sensitive to these targeted inhibitors and/or combinations in co-culture may require the use of novel agents with different target specificity. The newly developed MTOR inhibitors, such as MLN0128 (formerly called INK128) and Torin1, target the ATP binding site of MTOR. These inhibitors effectively suppress MTORC1-dependent 4EBP1 phosphorylation and MTORC2-dependent AKT activation; therefore, they block the functions of both MTOR complexes that are resistant to rapamycin and rapamycin analogs.46,47 We and others reported that these inhibitors are more potent than rapamycin and its analogs.35,47,48 Rahmani et al.42 recently reported that the combination of INK128 and ABT737 synergistically inhibited the growth of U937 cells in vitro and prolonged survival of leukemic mice. This study demonstrated an alternative strategy of replacing temsirolimus with these MTOR inhibitors in order to increase therapeutic efficacy, and warrant an investigation into the co-treatment of MTORC1/C2 inhibitor plus ABT737 or Nutlin-3a in primary samples under stromal co-culture.
Finally, our data support the notion of AML disease heterogeneity.49 Despite the variability in responses to the targeted inhibitors and their combinations among the 20 primary AML samples studied, distinct proteins/pathway alterations and apoptosis induction could correlate with unique disease characteristics. Likely due to the small sample size, results were largely negative except for associations with FLT3 mutation, one of the most common mutations in AML associated with poor prognosis. All four FLT3-mutated AML samples were less sensitive to ABT737 and instead responsive to the combination of temsirolimus plus ABT737 in stromal co-culture. Of note, we recently reported reduced sensitivity of FLT3-mutated AML to the selective BCL2 inhibitor ABT199 in a phase II AML clinical trial,32 possibly related to the reported upregulation of MCL1 protein conferring resistance to BCL2/BCL-XL inhibitors.50 In turn, MTOR inhibitors are capable of downregulating MCL1, especially under the condition mimicking BM microenvironment.42 A further validation study using a larger dataset of FLT3-mutated AML is needed to support these preliminary findings.
In summary, utilizing RPPA technology, we profiled AML signaling networks that are triggered by the target-selective inhibitors temsirolimus, ABT737, and Nutlin-3a and modified by stroma. We demonstrated that stroma-altered drug sensitivity is the result of signaling modifications and showed that exploring drug combinations in a co-culture model identifies the mechanism-based effective therapies to combat stroma-mediated AML survival. Altogether, our findings in this study provide important guidance for developing tailored treatments to eliminate microenvironment-protected AML.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/9/1537
Funding
This work was supported in part by National Institutes of Health/National Cancer Institute Leukemia SPORE Career Development Award P50 CA100632 (to ZZ); NIH/NCI grant 5 R01 CA155056-04 and Leukemia and Lymphoma Society grant 6427-13 (to MK); and NIH grants P01 CA55164, P30 CA016672, P50 CA100632, and R01 CA163481, a Cancer Prevention and Research Institute of Texas Shared Instrument Award, and the Paul and Mary Haas Chair in Genetics (all to MA).
References
- 1.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. [DOI] [PubMed] [Google Scholar]
- 2.Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood. 2016;127(1):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaffer BC, Gillet JP, Patel C, Baer MR, Bates SE, Gottesman MM. Drug resistance: still a daunting challenge to the successful treatment of AML. Drug Resist Updat. 2012;15(1–2):62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel C, Stenke L, Varma S, et al. Multidrug resistance in relapsed acute myeloid leukemia: evidence of biological heterogeneity. Cancer. 2013;119(16):3076–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: biology and therapeutic targeting. J Clin Oncol. 2011; 29(5):591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correia AL, Bissell MJ. The tumor microenvironment is a dominant force in multidrug resistance. Drug Resist Updat. 2012;15(1–2):39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker PS. Dependence of acute myeloid leukemia on adhesion within the bone marrow microenvironment. Scientific World Journal. 2012;2012:856467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min YH, Eom JI, Cheong JW, et al. Constitutive phosphorylation of Akt/PKB protein in acute myeloid leukemia: its significance as a prognostic variable. Leukemia. 2003;17(8):995–997. [DOI] [PubMed] [Google Scholar]
- 9.Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102(3):972–980. [DOI] [PubMed] [Google Scholar]
- 10.Martelli AM, Evangelisti C, Chiarini F, McCubrey JA. The phosphatidylinositol 3-kinase/Akt/mTOR signaling network as a therapeutic target in acute myelogenous leukemia patients. Oncotarget. 2010; 1(2):89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Konopleva M, Zhao S, Hu W, et al. The anti-apoptotic genes Bcl-X(L) and Bcl-2 are over-expressed and contribute to chemoresistance of non-proliferating leukaemic CD34+ cells. Br J Haematol. 2002; 118(2):521–534. [DOI] [PubMed] [Google Scholar]
- 12.Bueso-Ramos CE, Yang Y, deLeon E, McCown P, Stass SA, Albitar M. The human MDM-2 oncogene is overexpressed in leukemias. Blood. 1993;82(9):2617–2623. [PubMed] [Google Scholar]
- 13.Filipits M, Stranzl T, Pohl G, et al. Drug resistance factors in acute myeloid leukemia: a comparative analysis. Leukemia. 2000;14(1):68–76. [DOI] [PubMed] [Google Scholar]
- 14.Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16(9):1713–1724. [DOI] [PubMed] [Google Scholar]
- 15.Matsunaga T, Takemoto N, Sato T, et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med. 2003; 9(9):1158–1165. [DOI] [PubMed] [Google Scholar]
- 16.Hazlehurst LA, Argilagos RF, Dalton WS. Beta1 integrin mediated adhesion increases Bim protein degradation and contributes to drug resistance in leukaemia cells. Br J Haematol. 2007;136(2):269–275. [DOI] [PubMed] [Google Scholar]
- 17.Tabe Y, Jin L, Tsutsumi-Ishii Y, et al. Activation of integrin-linked kinase is a critical prosurvival pathway induced in leukemic cells by bone marrow-derived stromal cells. Cancer Res. 2007;67(2):684–694. [DOI] [PubMed] [Google Scholar]
- 18.Kojima K, McQueen T, Chen Y, et al. p53 activation of mesenchymal stromal cells partially abrogates microenvironment-mediated resistance to FLT3 inhibition in AML through HIF-1alpha-mediated down-regulation of CXCL12. Blood. 2011; 118(16):4431–4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shafer D, Grant S. Update on rational targeted therapy in AML. Blood Rev. 2016; 30(4):275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma WW, Jimeno A. Temsirolimus. Drugs Today (Barc). 2007;43(10):659–669. [DOI] [PubMed] [Google Scholar]
- 21.Zeng Z, Sarbassov dos D, Samudio IJ, et al. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood. 2007;109(8):3509–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435(7042):677–681. [DOI] [PubMed] [Google Scholar]
- 23.Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006; 10(5):375–388. [DOI] [PubMed] [Google Scholar]
- 24.Vassilev LT, Vu BT, Graves B, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303(5659):844–848. [DOI] [PubMed] [Google Scholar]
- 25.Kojima K, Konopleva M, Samudio IJ, et al. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood. 2005;106(9):3150–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teachey DT, Obzut DA, Cooperman J, et al. The mTOR inhibitor CCI-779 induces apoptosis and inhibits growth in preclinical models of primary adult human ALL. Blood. 2006;107(3):1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kojima K, Shimanuki M, Shikami M, et al. The dual PI3 kinase/mTOR inhibitor PI-103 prevents p53 induction by Mdm2 inhibition but enhances p53-mediated mitochondrial apoptosis in p53 wild-type AML. Leukemia. 2008;22(9):1728–1736. [DOI] [PubMed] [Google Scholar]
- 28.Kline MP, Rajkumar SV, Timm MM, et al. ABT-737, an inhibitor of Bcl-2 family proteins, is a potent inducer of apoptosis in multiple myeloma cells. Leukemia. 2007; 21(7):1549–1560. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Ruvolo VR, Gao C, et al. Evaluation of apoptosis induction by concomitant inhibition of MEK, mTOR, and Bcl-2 in human acute myelogenous leukemia cells. Mol Cancer Ther. 2014; 13(7):1848–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yee KWL, Garcia-Manero G, Thomas D, et al. A phase II study of temsirolimus (CCI-779) in patients with advanced leukemias. Blood. 2004;104(11):4523. [Google Scholar]
- 31.Andreeff M, Kelly KR, Yee K, et al. Results of the phase I trial of RG7112, a small-molecule MDM2 antagonist in leukemia. Clin Cancer Res. 2016;22(4):868–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konopleva M, Pollyea DA, Potluri J, et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016; 6(10):1106–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kornblau SM, Tibes R, Qiu YH, et al. Functional proteomic profiling of AML predicts response and survival. Blood. 2009; 113(1):154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itoh K, Tezuka H, Sakoda H, et al. Reproducible establishment of hemopoietic supportive stromal cell lines from murine bone marrow. Exp Hematol. 1989; 17(2):145–153. [PubMed] [Google Scholar]
- 35.Zeng ZH, Shi YX, Tsao T, et al. Targeting of mTORC1/2 by the mTOR kinase inhibitor PP242 induces apoptosis in AML cells under conditions mimicking the bone marrow microenvironment. Blood. 2012; 120(13):2679–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tibes R, Qiu Y, Lu Y, et al. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5(10):2512–2521. [DOI] [PubMed] [Google Scholar]
- 37.Hu J, He X, Baggerly KA, Coombes KR, Hennessy BTJ, Mills GB. Non-parametric quantification of protein lysate arrays. Bioinformatics. 2007;23(15):1986–1994. [DOI] [PubMed] [Google Scholar]
- 38.Neeley ES, Baggerly KA, Kornblau SM. Surface adjustment of reverse phase protein arrays using positive control spots. Cancer Inform. 2012;11:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim SH, Zukowski K, Novak RF. Rapamycin effects on mTOR signaling in benign, premalignant and malignant human breast epithelial cells. Anticancer Res. 2009;29(4):1143–1150. [PMC free article] [PubMed] [Google Scholar]
- 40.Matter ML, Ruoslahti E. A signaling pathway from the alpha5beta1 and alpha(v)beta3 integrins that elevates bcl-2 transcription. J Biol Chem. 2001; 276(30):27757–2763. [DOI] [PubMed] [Google Scholar]
- 41.Du W, Yi Y, Zhang H, et al. Rapamycin inhibits IGF-1-mediated up-regulation of MDM2 and sensitizes cancer cells to chemotherapy. Plos One. 2013;8(4):e63179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rahmani M, Aust MM, Hawkins E, et al. Co-administration of the mTORC1/TORC2 inhibitor INK128 and the Bcl-2/Bcl-xL antagonist ABT-737 kills human myeloid leukemia cells through Mcl-1 down-regulation and AKT inactiva tion. Haematologica. 2015;100(12):1553–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muranen T, Selfors LM, Worster DT, et al. Inhibition of PI3K/mTOR leads to adaptive resistance in matrix-attached cancer cells. Cancer Cell. 2012;21(2):227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cang S, Iragavarapu C, Savooji J, Song Y, Liu D. ABT-199 (venetoclax) and BCL-2 inhibitors in clinical development. J Hematol Oncol. 2015;8(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee MJ, Ye AS, Gardino AK, et al. Sequential application of anticancer drugs enhances cell death by rewiring apoptotic signaling networks. Cell. 2012;149(4):780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thoreen CC, Kang SA, Chang JW, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284(12):8023–8032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Janes MR, Vu C, Mallya S, et al. Efficacy of the investigational mTOR kinase inhibitor MLN0128/INK128 in models of B-cell acute lymphoblastic leukemia. Leukemia. 2013; 27(3):586–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng Z, Wang RY, Qiu YH, et al. MLN0128, a novel mTOR kinase inhibitor, disrupts survival signaling and triggers apoptosis in AML and AML stem/progenitor cells. Oncotarget. 2016;7(34):55083–55097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med 2015;373(12):1136–1152. [DOI] [PubMed] [Google Scholar]
- 50.Yoshimoto G, Miyamoto T, Jabbarzadeh-Tabrizi S, et al. FLT3-ITD up-regulates MCL-1 to promote survival of stem cells in acute myeloid leukemia via FLT3-ITD-specific STAT5 activation. Blood. 2009; 114(24):5034–5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.