In recent years gain-of-function driver mutations in JAK2, MPL and CALR have been identified that constitutively activate janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway signaling.1,2 Collectively, these mutations lead to the development of the vast majority of myeloproliferative neoplasms (MPNs), a group of related diseases including polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (MF). Patients with MPNs have higher mortality rates, primarily due to cardiovascular complications, infections and transformation to other hematological malignancies such as leukemias.3 They also suffer significant constitutional symptoms including pruritus, headaches, weight loss, loss of appetite, fatigue and night sweats. Current MPN therapeutics include venesection to control blood counts in PV, aspirin to reduce the risk of thrombosis in both PV and ET, and cytoreductive agents such as hydroxycarbamide to reduce blood counts. However, these treatments do not slow disease progression and provide little relief from sometimes debilitating constitutional symptoms. Recently, the JAK1/2 inhibitor ruxolitinib (rux) has emerged as a molecularly-targeted therapy option. In trials, rux delivers significant survival benefits to MF patients, as well as a decrease in spleen size and constitutional symptoms.4 Similarly, randomized trials in advanced PV patients also showed significant decreases in constitutional symptoms, spleen size and control of hematocrit in those receiving rux.5 However, while effective, access to rux remains restricted and it is not available to all patients who might benefit from its use. Given these limitations, access to more affordable JAK/STAT pathway inhibitors would potentially address a significant unmet clinical need.
We have previously identified methotrexate (MTX) as a dose-dependent inhibitor of STAT phosphorylation in JAK2 V617F positive erythroleukemia-derived HEL cells6 (Figure 1A). Originally developed as an anti-folate and chemotherapy agent, MTX was repurposed during the 1980’s at doses of around 1% of chemotherapy levels and is now widely used to treat multiple auto-immune and inflammatory diseases, including rheumatoid arthritis.7 Given that JAK/STAT pathway signaling is fundamental to both immune regulation and inflammatory responses8 we hypothesize that the effectiveness of MTX in these diseases is likely to be a consequence of JAK/STAT pathway suppression in vivo. As such, we reasoned that other diseases featuring inappropriate pathway activation may also respond to low-dose MTX treatment.
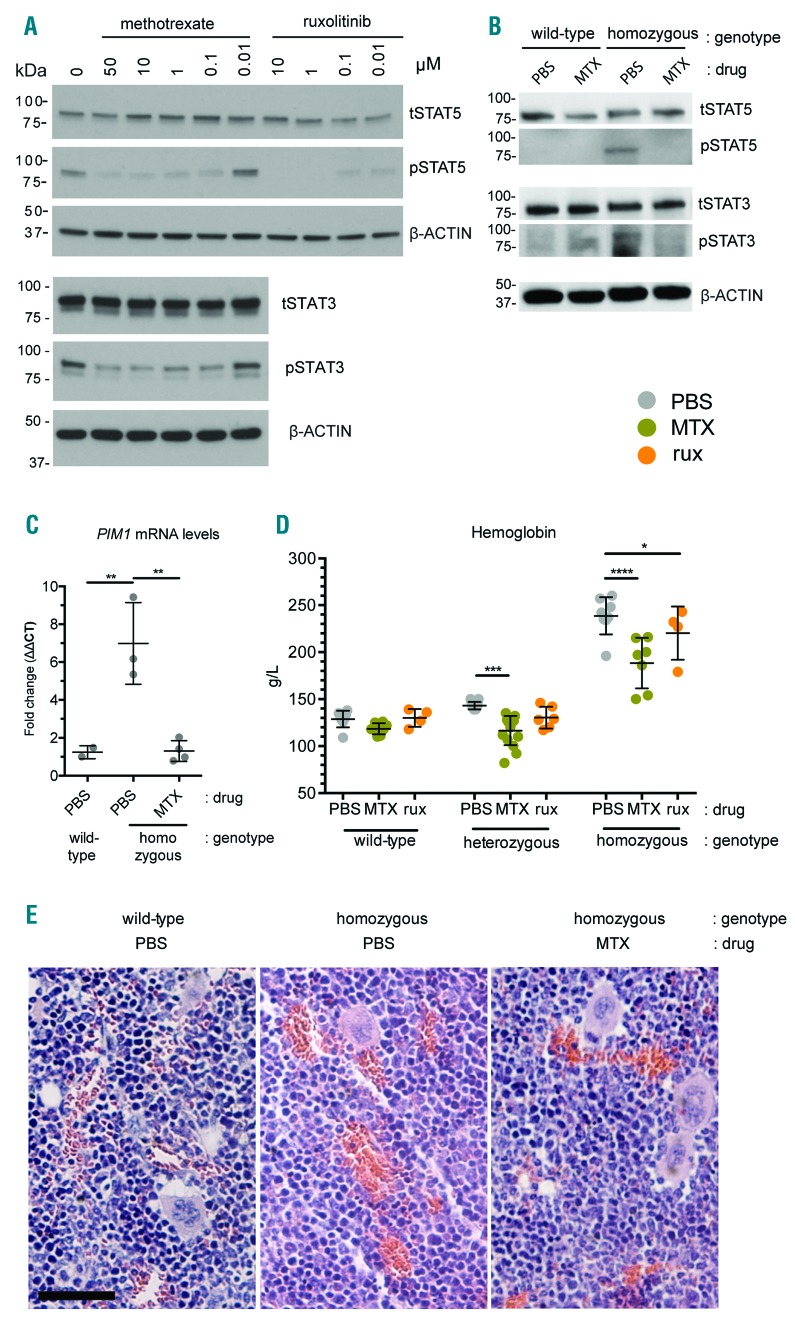
Figure 1.
Methotrexate is a JAK/STAT inhibitor that reduces hJAK2 V617F-induced erythrocytosis in vivo. (A,B) Western blots of the indicated total (t) and phosphorylated (p) STAT proteins in extracts from HEL cells (A) or the spleens of 10–11 week old mice of the indicated genotypes (B) following treatment with the indicated concentrations of MTX and rux. β-ACTIN serves as a loading control and apparent molecular weights are indicated in kDa. (C) Levels of PIM1 messenger ribonucleic acid (mRNA) expressed by spleen cells harvested from mice of the indicated genotype and drug treatments. Results are expressed as a fold change following normalization to β-actin mRNA and PBS-treated wild-type mice. (D) Hemoglobin concentration in blood from individual 10–11 week old mice of the indicated genotypes treated with either phosphate-buffered saline carrier control (PBS, gray), methotrexate (MTX, green) or ruxolitinib (rux, orange) for 28 days. Individual values, mean and standard deviations are shown. Samples were compared by one-way ANOVA. (E) Hematoxylin and eosin stained sections through decalcified tibia from mice of the indicated genotypes treated with the indicated compounds for 28 days. No marrow fibrosis was observed in either treated or untreated homozygous animals. Scale bar is 50μm. Images were obtained from a Zeiss Axioskop 2 with a 20×/0.5NA objective, a MicroPublisher 5.0 RTV camera and Qimaging v3.1.3.5 software. Brightness and contrast were adjusted in Photoshop CS5.
Consistent with its role in disease, transgenic mice whose endogenous JAK2 locus has been replaced by the human JAK2 V617F allele develop ET-like disease when heterozygous, and PV-like symptoms, including increased white cell counts (WCC), erythrocytosis and splenomegaly, when homozygous9 (Online Supplementary Figure S1). We therefore treated wild-type and hJAK2 V617F age-matched littermates with either MTX or vehicle control phosphate-buffered saline (PBS) for 28 days using a low-dose regime previously shown to reduce rheumatoid arthritis-like symptoms in mouse models.10,11 Compared to controls, spleens of homozygous hJAK2 V617F mice contain increased levels of phosphorylated (p)STAT5 and pSTAT3 (Figure 1B) while messenger ribonucleic acid (mRNA) levels of the JAK2/STAT5 target gene PIM1 are also increased (Figure 1C). Strikingly, and consistent with in vitro results, homozygotes treated with MTX have reduced levels of pSTAT5 and pSTAT3 (Figure 1B) and express significantly lower levels of the pathway target gene PIM1 (Figure 1C), suggesting that low-dose MTX also inhibits the JAK/STAT pathway in vivo.
We next tested hJAK2 V617F-expressing mice treated with MTX or rux as a positive control.12 Increased hemoglobin levels in both heterozygous and homozygous hJAK2 V617F mice are significantly reduced following treatment with MTX (Figure 1D), as are red blood cell counts and hematocrit levels (Online Supplementary Figure S2A,B). Importantly, despite being as effective as rux under these conditions, MTX treatment does not result in the general myelosuppression of wild-type controls (Figure 1D, Online Supplementary Figure S2), and both controls and MTX-treated mice continued to gain weight throughout the course of the experiment (data not shown). While platelet numbers and mean corpuscular volume are not significantly affected (Online Supplementary Figure S2C,D), MTX treatment is sufficient to normalize WCC in both heterozygous and homozygous hJAK2 V617F mice, but does not affect the differentiation or relative levels of individual white blood cell subtypes (Online Supplementary Figure S3). Consistent with these findings, histological analysis of bone marrows (Figure 1E and Online Supplementary Figure S4) shows no reduction in cellularity or differentiation in any of the three hematopoietic lineages in MTX-treated animals. Homozygous animals do, however, have erythrocytic and megakaryocytic hyperplasia and polylobated nuclei in a proportion of megakaryocytes, as previously described,9 phenotypes that are reduced in MTX-treated animals. As such, bone marrow morphology is consistent with a disease-specific effect of MTX, rather than nonspecific myelosuppression.
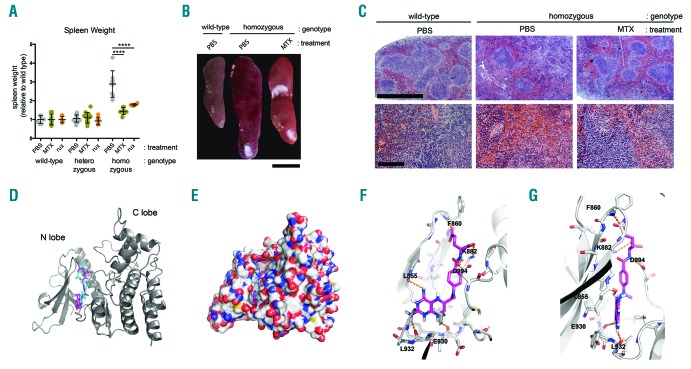
Another MPN-related phenotype, and cause of considerable constitutional symptoms in patients, is splenomegaly.13 By 10–11 weeks of age homozygous hJAK2 V617F mice have spleens that are around 3 times larger than their wild-type littermates, an enlargement that is strongly reduced by treatment with both MTX and rux (Figure 2A,B). Histological analysis of these spleens shows that homozygotes have erythroid hyperplasia with red pulp expansion and loss of normal architecture, with occasional megakaryocyte clusters,9 features that are mitigated by MTX treatment which results in reduced erythroid infiltration and a correspondingly improved morphology of white pulp (Figure 2C and Online Supplementary Figure S5).
Figure 2.
Methotrexate reduces splenomegaly. (A) Weight of individual spleens from 10–11 week old mice of the indicated genotypes shown relative to wild-type controls treated with phosphate-buffered saline carrier control (PBS, gray), methotrexate (MTX, green) or ruxolitinib (rux, orange) for 28 days. Samples were compared by one-way ANOVA. (B) Representative spleens from mice of the indicated genotypes and treatments immediately after dissection showing differences in size and reduction in spleen size following MTX treatment. Scale bar is 5mm. (C) Hematoxylin and eosin stained sections through formalin-fixed spleens from mice of the indicated genotypes treated with the indicated compounds for 28 days. Images were obtained from a Zeiss Axioskop 2 with 5×/0.15NA (top row, scale bar is 1mm) and 20×/0.5NA (lower row, scale bar is 100μm) objectives, a MicroPublisher 5.0 RTV camera and Qimaging v3.1.3.5 software. Brightness and contrast were adjusted in Photoshop CS5. (D–G) Predicted interactions of methotrexate with JAK2 occludes the ATP-binding site. (D) Illustrated representation of the human JAK2 JH1 (kinase) domain (from 5TQ8.pdb). Methotrexate (magenta sticks) is predicted to bind between the N and C lobes of the kinase in the ATP-binding site (cyan sticks). (E) Molecular surface of kinase with ligands bound (carbon gray, nitrogen blue and oxygen atoms shown in red). (F) MTX showing predicted H-bonding (orange dashes) to labelled residues within the binding site and ion-pair interaction with lysine 882. Residues highlighted in F and G are within 5 Å of the bound ligand. (G) View of bound MTX as in (F) rotated 90° about the Y axis.
Although MTX treatment reduces spleen weight and reticulocyte numbers in JAK2 V617F homozygous mice (Online Supplementary Figure S6A,B), we also observed modest increases in reticulocyte numbers and spleen size in wild-type littermates (Online Supplementary Figure S6A,B). Although the precise basis of these phenotypes is unclear, it is possible that splenic enlargement in wild-type mice is associated with hypersplenism and increased red cell destruction. In this scenario, the elevated reticulocyte count is notable as it supports a model in which methotrexate is not causing myelosuppression, with the marrow being able to increase erythropoiesis and mount an appropriate reticulocyte response. Intriguingly, we also observed slight increases in pSTAT5 and PIM1 mRNA levels in MTX-treated wild-type animals (Online Supplementary Figure S6C,D), changes that are not mirrored in JAK2 V617F homozygotes. While a detailed molecular analysis of the interactions between MTX and wild-type JAK2 will be needed to elucidate the basis of these observations, it is possible that this observation may be relevant to the rare cases of MTX-induced lymphoma previously described in rheumatoid arthritis patients.14
Finally, in order to gain insight into the potential mechanistic basis of MTX-mediated effects observed in vivo, we undertook an in silico study whereby a high resolution structure of the human JAK2 JH1 kinase domain15 was used as a target onto which to dock MTX and adenosine triphosphate (ATP; Figure 2D–G, Online Supplementary Table S1). Although such studies can only ever be indicative of potential interactions, it is intriguing to note that MTX is predicted to occupy the ATP binding pocket of the kinase with a binding affinity higher than ATP itself, suggesting that MTX may potentially be acting directly as a Type 1 kinase inhibitor in vivo. Taken together, our results show that low-dose MTX not only acts as an inhibitor of JAK/STAT signaling in vivo, but also strongly reduces the hematological phenotypes and splenomegaly associated with this hJAK2 V617F-based mouse model of human MPNs. Moreover, these effects are not a consequence of drug-induced myelosuppression. While JAK/STAT pathway activity has clearly been reduced in these JAK2 V617F mice, it remains a possibility that at least some of the disease-related responses observed are a consequence of reduced systemic inflammation mediated by MTX. Interestingly, previous studies have also demonstrated striking reductions in the levels of pro-inflammatory biomarkers such as C-Reactive protein, tumor necrosis factor α and interleukin 6 in human MF patients treated with rux.4 However, the link between MPN-related phenotypes and inflammatory markers remains to be elucidated.
Although not curative, rux is clinically valuable in multiple MPN patient populations, reducing mortality, normalizing hematological values and strongly reducing constitutional symptoms.4,5,13 However, concerns regarding cost-effectiveness result in limited availability even in well-funded healthcare systems. By contrast, MTX is a low-cost generic on the World Health Organization list of essential drugs. It is routinely prescribed for millions of patients worldwide and has a well understood toxicology and safety profile. Herein we have shown that low-dose MTX suppresses JAK/STAT pathway activity in vivo and is able to normalize hematological and splenic hyperplasia in mouse models of human MPNs. Consistent with our results, a recent case study has demonstrated significant hematological and symptomatic improvements in two Italian MPN patients following low-dose MTX treatment.16 In light of these results, we suggest that clinical trials should be undertaken to assess the safety and efficacy of low-dose MTX as a JAK/STAT inhibitor in human myeloproliferative neoplasms. If results are promising, repurposing MTX has the potential to provide a new, molecularly targeted treatment for MPN patients within a budget accessible to healthcare systems throughout the world - a development that may ultimately provide substantial clinical and health economic benefits.
Supplementary Material
Acknowledgments
All authors would like to thank Rachel Rodham, Matt Fischer and Anne Fowles, for technical help with this mouse study. Ben Orrah at the RHH hematology diagnostic labs and Darren Lath for help with histology.
Footnotes
Funding: AG was supported by the Wellcome Trust, the Medical Research Council, Bloodwise and Cancer Research UK. Funding was provided by a Senior Cancer Research UK fellowship to MZ, a Cancer Research UK / Yorkshire Cancer Research clinical PhD fellowship to ST, support from the Sheffield Blood Cancers Charity (MZ & AC) and an MRC Confidence in Concept award to MZ.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–1061. [DOI] [PubMed] [Google Scholar]
- 2.Vainchenker W, Constantinescu SN, Plo I. Recent advances in understanding myelofibrosis and essential thrombocythemia. F1000Res. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hultcrantz M, Wilkes SR, Kristinsson SY, et al. Risk and cause of death in patients diagnosed with myeloproliferative neoplasms in Sweden between 1973 and 2005: a population-based study. J Clin Oncol. 2015;33(20):2288–2295. [DOI] [PubMed] [Google Scholar]
- 4.Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vannucchi AM, Kiladjian JJ, Griesshammer M, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015;372(5):426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas S, Fisher KH, Snowden JA, Danson SJ, Brown S, Zeidler MP. Methotrexate Is a JAK/STAT Pathway Inhibitor. PLoS One. 2015;10(7):e0130078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bookbinder SA, Espinoza LR, Fenske NA, Germain BF, Vasey FB. Methotrexate: its use in the rheumatic diseases. Clin Exp Rheumatol. 1984;2(2):185–193. [PubMed] [Google Scholar]
- 8.Villarino AV, Kanno Y, Ferdinand JR, O’Shea JJ. Mechanisms of Jak/STAT signaling in immunity and disease. J Immunol. 2015;194(1):21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J, Kent DG, Godfrey AL, et al. JAK2V617F homozygosity drives a phenotypic switch in myeloproliferative neoplasms, but is insufficient to sustain disease. Blood. 2014;123(20):3139–3151. [DOI] [PubMed] [Google Scholar]
- 10.Lange F, Bajtner E, Rintisch C, Nandakumar KS, Sack U, Holmdahl R. Methotrexate ameliorates T cell dependent autoimmune arthritis and encephalomyelitis but not antibody induced or fibroblast induced arthritis. Ann Rheum Dis. 2005;64(4):599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joly MS, Martin RP, Mitra-Kaushik S, et al. Transient low-dose methotrexate generates B regulatory cells that mediate antigen-specific tolerance to alglucosidase alfa. J Immunol. 2014;193(8):3947–3958. [DOI] [PubMed] [Google Scholar]
- 12.Quintas-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115(15):3109–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mesa R, Verstovsek S, Kiladjian JJ, et al. Changes in quality of life and disease-related symptoms in patients with polycythemia vera receiving ruxolitinib or standard therapy. Eur J Haematol. 2016;97(2):192–200. [DOI] [PubMed] [Google Scholar]
- 14.Salloum E, Cooper DL, Howe G, et al. Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clin Oncol. 1996;14(6):1943–1949. [DOI] [PubMed] [Google Scholar]
- 15.Jones P, Storer RI, Sabnis YA, et al. Design and synthesis of a panjanus kinase inhibitor clinical candidate (PF-06263276) suitable for inhaled and topical delivery for the treatment of inflammatory diseases of the lungs and skin. J Med Chem. 2017;60(2):767–786. [DOI] [PubMed] [Google Scholar]
- 16.Palandri F, Labate C, Sabattini E, Catani L, Martino B. Low-dose methotrexate as treatment of myeloproliferative neoplasms: Proof of principle of clinical activity. [letter]. Am J Hematol. 2016;91(8):E329–330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.