Figure 2.
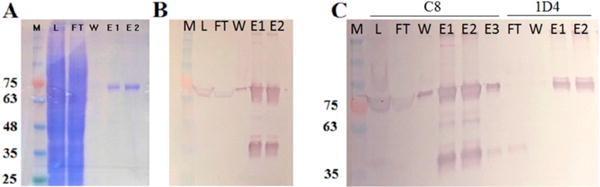
SDS–PAGE and Western blot analysis of fractions from immunoaffinity purification of RhoPDE from HEK293 cells. (A) Coomassie-stained gel and (B) C8-probed Western blot of C8-purified RhoPDE: lane M, molecular mass markers in kilodaltons; lane L, load, postnuclear supernatant fraction; lane FT, flow-through, not bound to the C8 column; lane W, final wash before the first elution with the C8 peptide; lane E1, first elution with the C8 peptide; lane E2, second elution with the C8 peptide. (C) C8-probed Western blot of tandem C8- and 1D4-purified RhoPDE. Lanes from left to right: lane M, molecular mass markers; lane L, load, postnuclear supernatant fraction; lane FT, flow-through, not bound to the C8 column; lane W, final wash before the first elution with the C8 peptide; lane E1, first elution with the C8 peptide; lane E2, second elution with the C8 peptide; lane E3, third elution with the C8 peptide; lane FT, flow-through, nonbound fraction from E1–E3 from the C8 column applied to the 1D4 column; lane W, final wash before the first elution with the 1D4 peptide; lane E1, first elution with the 1D4 peptide; lane E2, second elution with the 1D4 peptide. Note that these experiments were optimized to yield a high concentration of RhoPDE in the final eluate and not for a high yield of protein from the HEK293 cells. Thus, the C8 column was overloaded in these examples where excess RhoPDE can be observed in Western blots of the FT lanes. In addition, it must be kept in mind that the Western blots in this figure are significantly overloaded with protein levels well outside of the linear detection range (the same amount of material was loaded on the Western blots as was loaded on the Coomassie-stained gel). This can be seen most readily from the truncated Rho domain (migrating at approximately 35–40 kDa) observed in both Western blots of panels B and C, but not visible in the Coomassie-stained gel in panel A.
