Figure 5.
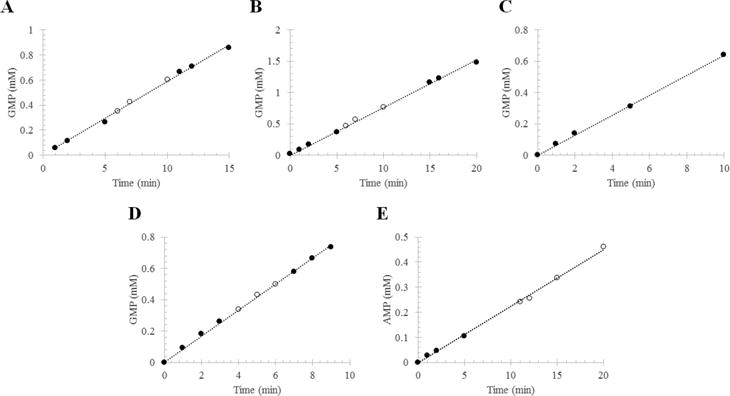
Phosphodiesterase activity of RhoPDE from transiently transfected HEK293-GnT1− cells. The reaction mixture contained RhoPDE in membranes isolated from transfected cells or in liposomes reconstituted with protein that had been purified from transfected cells. The RhoPDE used in this figure contained only the C-terminal 1D4 tag (i.e., no C8 tag). The formation of 5′-GMP from cGMP (or 5′-AMP from cAMP) was followed by HPLC: (●) reaction in the dark and (○) reaction in the light. Integrated peaks corresponding to GMP (or AMP) at each time point were converted into concentrations with a standard curve and plotted vs time. Panels A and B show two representative reactions with RhoPDE (50 nM) in HEK293 membranes reconstituted with ATR. (A) kcat = 19.6 ± 0.2 s−1; (B) kcat = 25.4 ± 0.2 s−1. (C) Reaction with RhoPDE (50 nM) in HEK293 membranes not reconstituted with ATR. This reaction was performed in the absence of light from the 300 W tungsten source. kcat = 21.3 ± 0.2 s−1. (D) cGMP phosphodiesterase actvity of RhoPDE (50 nM) in reconstituted liposomes. kcat = 27.7 ± 0.2 s−1. (E) cAMP phosphodiesterase actvity of RhoPDE (100 nM) in reconstituted liposomes. kcat = 3.7 ± 0.1 s−1.
