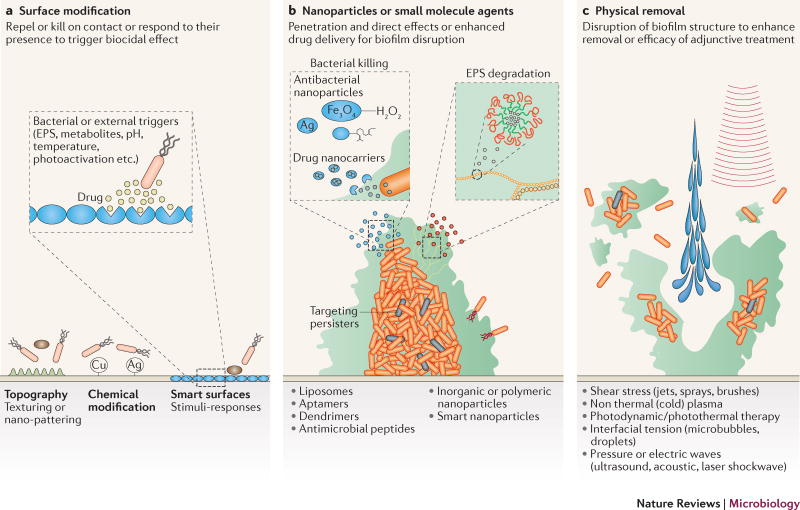
Figure 3. Technological approaches to combat biofilms.
Recent advances in material science and nanotechnology enabled the engineering of a wide array of biofilm-targeting strategies. a) The material and surface properties of medical devices, such as surface charge, hydrophobicity, roughness, topography and chemistry among others, can be modified to prevent bacterial attachment and therefore attenuate or block biofilm formation. Additionally, ‘smart’ or stimuli-triggered responsive surfaces can be constructed that elicit their effect only in response to physical contact with cell-wall or membrane associated adhesins or chemical cues (i.e. secreted EPS, metabolites) of the bacteria. b) Advancement in nanoparticle synthesis has led to the development of diverse approaches to combat biofilms. Inorganic metallic (silver, copper etc.) and organic nanoparticles (liposomes, aptamers etc.), have been increasingly evaluated to improve their anti-biofilm efficacy, as well as their biocompatibility to reduce toxic effects on the host. Nanoparticles can be used to form nanocoatings, be incorporated into materials as composites or fillings or combined together with conventional antimicrobials and other approaches designed to physically disrupt or remove the biofilm. Furthermore, antimicrobial peptides (AMPs) and aptamers also display specific biofilm-targeting properties that can be also used to enhance specificity and efficacy of nanoparticles (hybrid nanoparticles). c) New technologies for physical biofilm removal, including mechanical, energy- and light-based disruption, may further improve biofilm intervention strategies. Given the multifaceted nature of biofilm formation and the complex microbial interactions with the surrounding physical and chemical environment, a combination of these approaches may be required to successfully combat biofilm-mediated disease.