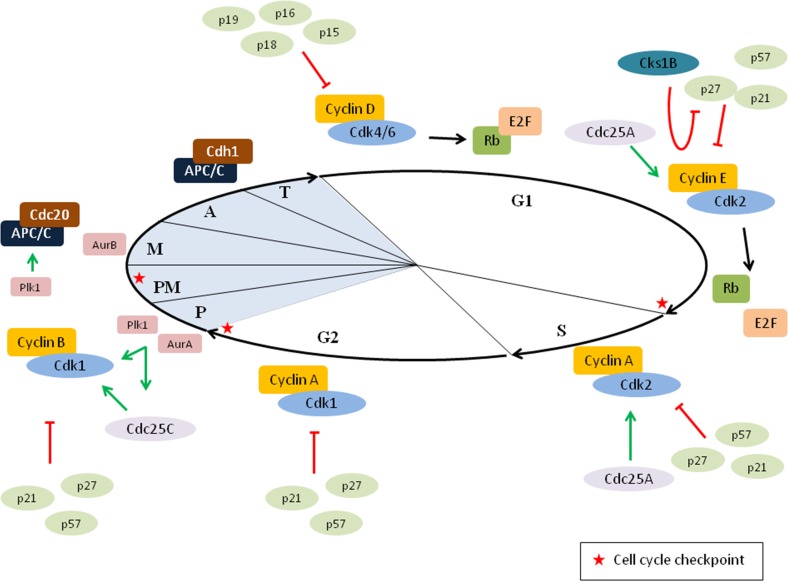
Figure 2. Cell cycle regulation.
Cell cycle progression is highly regulated by the coordinated activation of cyclin–cyclin-dependent kinases (Cdk) dimers. In early G1, cyclin D–Cdk4/6 complexes are formed and phosphorylate the retinoblastoma protein (Rb). Phosphorylation of Rb results in the release of the transcription factor E2F, thus enabling transcription of early E2F response genes. In late G1, Rb is further phosphorylated by cyclin E–Cdk2, allowing further transcription of E2F responsive genes and passage through the G1/S restriction point. In addition, Cks1B facilitates the p27 degradation by forming a bridge between Cdk2-cyclinE–p27 and Skp2. Once the cells are in S phase, cyclin A–Cdk2 complexes are formed. At the end of the interphase, cyclin A–Cdk1 is activated to initiate the mitosis and the progression through the mitosis depends on cyclin B–Cdk1. Importantly, the function of these cyclin-Cdk dimers can be modulated by either activators (Cdc25A and Cdc25C) or inhibitors (INK4 family and Kip/Cip family of Cdk inhibitors). In early mitosis, aurora A kinase (AurA) and polo-like kinase 1 (Plk1) promote the activation of cyclin B–Cdk1 complexes. In addition, both Plk1 and AurA can, directly or indirectly, stimulate the activator Cdc25C. During prometaphase the spindle assembly checkpoint (SAC) pathway is blocking the activation of the anaphase-promoting complex/cyclosome (APC/C). However, once bi-orientation is achieved the APC/C is activated by Cdc20 allowing degradation of cyclin B. The formation of APC/C-Cdc20 is also stimulated by Plk1. In late mitosis, APC/C is activated by Cdh1 and important mitotic proteins are degraded. P: prophase, PM: prometaphase, M: metaphase, A: anaphase and T: telophase.