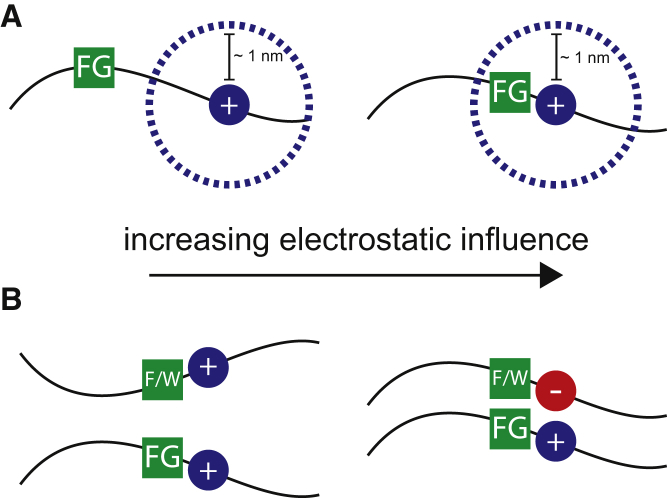
Figure 8.
Schematic of the influence of electrostatics on hydrophobic interactions mediated by FG domains. (A) At physiologically relevant salt concentrations, electrostatic interactions typically have a range of ∼1 nm (Debye length, dotted lines), which determines how much a charged residue influences the FG sequence’s recognition of and binding to hydrophobic substrates. When charged residues are moved further away from the FG sequence, electrostatic interactions become less significant for FG-mediated self-assembly and selective transport. (B) Complementary charge and hydrophobic interactions enable the strongest interactions between a substrate and FG domains. Modulating charge types and the presence of hydrophobic domains can tune interactions from strong binding to free diffusion within the gel and in in vivo systems. To see this figure in color, go online.