Abstract
AIM
To investigate the value of the gamma-glutamyltraspeptidase (GGT)-to-platelet (PLT) ratio (GPR) in the diagnosis of hepatic fibrosis in patients with chronic hepatitis B (CHB).
METHODS
We included 390 untreated CHB patients in this study. The GPR, aspartate aminotransferase (AST)-to-PLT ratio index (APRI), and fibrosis-4 (FIB-4) of all patients were analysed to determine if these parameter were correlated with age, gender, medical history, liver function [total bilirubin (TBil), alanine aminotransferase (ALT), and AST], GGT, PLT count, or hepatic fibrosis stage. The GPR, APRI, and FIB-4, as well as the combination of the GPR and APRI or the GPR and FIB-4 were assessed in different cirrhosis stages using receiver operating characteristic (ROC) curve analysis to evaluate their value in diagnosing hepatic fibrosis in CHB patients.
RESULTS
The GPR, APRI, and FIB-4 were not correlated with CHB patients’ age, gender, or disease duration (P > 0.05), but all of these parameters were positively correlated with serum ALT, AST, GGT, and PLT count (P < 0.01). Additionally, the GPR, APRI, and FIB-4 were positively correlated with hepatic fibrosis (P < 0.01); the areas under the ROC curve for the GPR in F1, F2, F3, and F4 stages were 0.723, 0.741, 0.826, and 0.833, respectively, which were significantly higher than the respective values for the FIB-4 and APRI (F1: 0.581, 0.612; F2: 0.706, 0.711; F3: 0.73, 0.751; and F4: 0.799, 0.778). The respective diagnostic cut-off points for each stage were 0.402, 0.448, 0.548, and 0.833, respectively. The diagnostic sensitivity and specificity were, respectively, 88.8% and 87.5% in F1, 72.7% and 89.7% in F2, 81.3% and 98.6% in F3, and 80% and 97.4% in F4 when the GPR and APRI were connected in parallel; 86.6% and 90.2%, 78.4% and 96%, 78.6% and 97.4%, and 73.2% and 97.9%, respectively, when the GPR and APRI were connected in series; 80.2% and 89%, 65% and 89%, 70.3% and 98.5%, and 78.8% and 96.8%, respectively, when the GPR and FIB-4 were connected in parallel; and 83.6% and 87.9%, 76.8% and 96.6%, 72.7% and 98%, and 74.4% and 97.7%, respectively, when the GPR and FIB-4 were connected in series.
CONCLUSION
The GPR, as a serum diagnostic index of liver fibrosis, is more accurate, sensitive, and easy to use than the FIB-4 and APRI, and the GPR can significantly improve the sensitivity and specificity of hepatic fibrosis diagnosis in CHB when combined with the FIB-4 or APRI.
Keywords: Gamma-glutamyltraspeptidase-to-platelet ratio, APRI, FIB-4, Chronic hepatitis B, Hepatic fibrosis
Core tip: Hepatic fibrosis is a precursor of cirrhosis for chronic hepatitis B patients. Severe hepatic fibrosis and cirrhosis can increase the incidence and mortality of primary liver cancer. Although liver biopsy is still the gold standard for the diagnosis of liver fibrosis, it is not widely used as a routine examination because of its invasiveness, high cost, and lack of repeatability. Identification of a simple, convenient, cheap, and noninvasive index to assess patients’ hepatic fibrosis level is thus urgently needed. The aim of the present study was to investigate the value of the gamma-glutamyltranspeptidase-to-platelet ratio in the diagnosis of hepatic fibrosis in patients with chronic hepatitis B.
INTRODUCTION
Hepatic fibrosis is a precursor of cirrhosis in patients with chronic hepatitis B (CHB)[1]. Severe hepatic fibrosis and cirrhosis can increase the incidence and mortality of primary liver cancer[2]. Although liver biopsy is still the gold standard for the diagnosis of liver fibrosis, it is not widely used as a routine examination because of its invasiveness, high cost, and lack of repeatability. The aspartate aminotransferase (AST)-to-platelet (PLT) ratio index (APRI) and fibrosis-4 (FIB-4) are commonly used in the clinic as two noninvasive serum models; but their complicated calculation, poor sensitivity, and requirement for combination with other indexes for comprehensive evaluation of the degree of hepatic fibrosis actually increase the workload. As an alternative, transient elastography (Fibroscan) by imaging is considered a good tool for the diagnosis of hepatic fibrosis, but its performance is restricted by several factors, such as diet, obesity, ascites, and rib gap width. In recent years, researchers have tried to identify a simple, convenient, cheap, and noninvasive index to assess patients’ hepatic fibrosis level, which could assist in early diagnosis to achieve timely treatment, delay cirrhosis or liver cancer incidence, improve patients’ quality of life, and prolong patients’ survival time.
The gamma-glutamyltraspeptidase (GGT)-to-PLT ratio (GPR) is a newly reported model for evaluating the grade of hepatic fibrosis, which is of great value in predicting hepatic fibrosis[3-11]. In June 2015, Lemoine et al[3] first reported that the GPR could be widely used as an independent predictor to assess hepatic fibrosis in CHB patients in West Africa, and that the sensitivity of the GPR is higher than that of the APRI and FIB-4. In November 2015, Boyd et al[9] showed that in patients with HBV and HIV superinfection in France, the GPR can predict the level of significant hepatic fibrosis. In November 2016, Li et al[11] reported that GPR was better than other noninvasive serum models in assessing hepatic fibrosis in CHB patients with high HBV DNA (≥ 5 log10 copies/mL) and normal or mildly elevated alanine transaminase (ALT) [≤ 2 times upper limit of normal (ULN)] in a Chinese population. Moreover, Lemoine et al[4] and Park et al[5] each reported that the GPR could be used as an independent factor in the preoperative evaluation of patients with primary liver cancer caused by CHB. However, likely due to the small sample size, they did not carry out an in-depth stratified pathological study of hepatic fibrosis. Based on these findings, to further explore the value of the GPR in the diagnosis of hepatic fibrosis, we retrospectively analysed a total of 652 outpatients and inpatients diagnosed with CHB at the General Hospital of Ningxia Medical University from May 2010 to January 2016, among whom 390 newly diagnosed CHB patients with complete data without previous therapy were examined by correlation analysis and receiver operating characteristic curve (ROC) analysis. The correlations among patient’s general information, medical history, and laboratory examination results were determined.
MATERIALS AND METHODS
Case selection
There were 652 outpatients and inpatients diagnosed with CHB at the General Hospital of Ningxia Medical University from May 2010 to January 2017. Patients with severe heart, brain or kidney disease; severe infection; super infection with hepatitis A virus, hepatitis C virus, hepatitis D virus, or hepatitis E virus or autoimmune liver disease; heavy drinking; or pregnancy were excluded. The remaining 390 newly diagnosed CHB patients, who did not undergo hepatoprotective, anti-hepatic fibrosis drug or antiviral drug treatments, were selected for the study, which included 283 males and 107 females. All CHB patients in the study had a complete medical history; data on HBV serum markers, serum HBV DNA, and liver function; and liver biopsy results. According to the clinical diagnostic criteria in the Guidelines for prevention and treatment of chronic hepatitis B published in 2001, all of the CHB patients were classified and analysed based on liver function and liver fibrosis.
Clinical indexes
The clinical characteristics of the CHB patients in this study, including their medical history, HBV serum markers, liver function, blood platelets, serum GGT, hepatic fibrosis indexes (APRI, FIB-4, and GPR), and pathological grade of hepatic fibrosis, were statistically analysed.
HBV serum marker detection: Chemiluminescence detection was used to assess HBV serum markers. The equipment and reagents were provided by Abbott Company (United States).
Liver function and GGT detection: A kinetic method was used to assess liver function and GGT. An ACX9 automatic biochemical analyser (Beckman Company, United States) was employed for this purpose. The biochemical test kit was also from Beckman. GGT normal range was 10-60 U/L.
Platelet detection: An automatic blood cell analyser was used for platelet detection. The normal range was 100-300 × 109/L.
APRI and FIB-4 calculation formulas: The formulas for calculating the APRI and FIB-4 indexes were as follows: APRI = (AST/ULN) × 100/PLT count (109/L) and FIB-4 = (age × AST)/(PLT count × ALT square root). Total bilirubin (TBIL) normal range was 3.4-17.1 μmol/L. AST normal range was 15-40 U/L. ALT normal range was 9-50 U/L.
Hepatic tissue pathological fibrosis analysis: The degree of fibrosis was graded from F1 to F4 according to the guidelines on CHB published in 2015.
Statistical method: A database of all data was established in Excel2000. The data, expressed as the mean ± SD, were statistically analysed using SPSS17.0 software. Normally distributed data were compared by t-test, and non-normally distributed data were compared using the χ2 test. In addition, correlations were determined by Pearson correlation analysis. P < 0.05 indicated that the difference was statistically significant. ROC curves were plotted for the GPR, APRI and FIB-4; and the areas under the curve (AUCs) were calculated to determine the optimal cut-off points on the ROC curves corresponding to the greatest sum of the sensitivity and specificity and to calculate the rate of diagnostic accuracy. ROC curve is a graphical plot that illustrates the diagnostic ability of a binary classifier system as its discrimination threshold varies. The ROC curve is created by plotting the true positive rate (TPR) against the false positive rate (FPR) at various threshold settings. The TPR is also known as sensitivity, recall or probability of detection in machine learning. The FPR is also known as the fall-out or probability of false alarm and can be calculated as (1 - specificity). The ROC curve is thus the sensitivity as a function of fall-out. In general, if the probability distributions for both detection and false alarm are known, the ROC curve can be generated by plotting the cumulative distribution function of the detection probability on the Y-axis vs the cumulative distribution function of the false-alarm probability on the x-axis.
The value of the GPR, APRI, and FIB-4, as well as the combination of the GPR and APRI or the GPR and FIB-4, in the diagnosis of CHB associated liver fibrosis was evaluated according to the test results. A difference was considered statistically significant at P < 0.05.
RESULTS
Correlation analysis of GPR, APRI, and FIB-4 and clinical data
A total of 390 patients with CHB were enrolled in this study, including 283 males and 107 females, with a mean age of 38.94 ± 11.39 years. Pearson correlation analysis showed that the GPR, APRI and FIB-4 were not associated with the CHB patients’ age, gender, or the disease course, but were associated with TBil, AST, ALT, GGT, and PLT count (P < 0.01; Table 1).
Table 1.
Correlation of the GPR, APRI, and FIB-4 (mean ± SD) with the baseline characteristics of patients
| Parameter |
GPR |
APRI |
FIB-4 |
||
| Mean ± SD | r | r | r | P | |
| TBIL (μmol/L) | 22.70 ± 28.53 | 0.275 | 0.205 | 0.258 | < 0.01 |
| AST (IU/L) | 81.71 ± 146.11 | 0.183 | 0.644 | 0.834 | < 0.01 |
| ALT (IU/L) | 125.71 ± 246.43 | 0.113 | 0.547 | 0.764 | < 0.01 |
| GGT (IU/L) | 225.21 ± 1008.88 | 0.779 | 0.222 | 0.235 | < 0.01 |
| PLT count (109/L) | 160.82 ± 66.61 | -0.285 | -0.285 | -0.26 | < 0.01 |
| GPR | 0.73 ± 1.79 | 1.000 | 0.385 | 0.255 | < 0.01 |
| APRI | 65.44 ± 140.77 | 0.385 | 1.000 | 0.847 | < 0.01 |
| FIB-4 | 417.93 ± 1367.85 | 0.255 | 0.847 | 1.000 | < 0.01 |
TBIL: Total bilirubin; AST: Aspartate transaminase; ALT: Alanine transaminase; GGT: Gamma-glutamyltranspeptidase; PLT: Platelet; GPR: the gamma-glutamyltranspeptidase-to-platelet ratio; APRI: (AST)-to-platelet ratio index; FIB-4: Fibrosis-4.
Correlation analysis of GPR, APRI, and FIB-4 and CHB liver function classification
To investigate the relationships between the GPR, APRI and FIB-4 and CHB disease, the CHB patients were divided into three groups according to their condition: 244 mild cases, 57 moderate cases, and 80 severe cases. Spearman correlation analysis showed that the GPR, APRI, and FIB-4 were positively related to the grade of the CHB patients’ liver function (P < 0.01; Table 2).
Table 2.
Correlation of the GPR, APRI, and FIB-4 (mean ± SD) with the liver function severity classification
| Parameter | n | GPR | APRI | FIB-4 |
| Liver function grade mild | ||||
| Mild | 244 | 0.50 ± 0.92 | 30.71 ± 76.73 | 68.39 ± 126.12 |
| r | 0.213 | 0.091 | 0.219 | |
| P | < 0.01 | < 0.01 | < 0.01 | |
| Moderate | 57 | 1.57 ± 3.59 | 101.20 ± 202.09 | 511.23 ± 1234.61 |
| r | 0.11 | 0.16 | 0.191 | |
| P | < 0.01 | < 0.01 | < 0.01 | |
| Severe | 80 | 0.84 ± 1.74 | 145.90 ± 192.51 | 1417.54 ± 2544.58 |
| r | 0.016 | 0.563 | 0.720 | |
| P | < 0.01 | < 0.01 | < 0.01 |
Correlation analysis of GPR, APRI, and FIB-4 and hepatic fibrosis stages of CHB
According to the liver biopsy results, the degrees of hepatic fibrosis was divided into F1 to F4, and the Spearman rank correlation analysis showed that the GPR, APRI, and FIB-4 were positively correlated with the stage of hepatic fibrosis (P < 0.01; Table 3).
Table 3.
Correlation of the GPR, APRI, and FIB-4 (mean ± SD) with the fibrosis grade
| Parameter | n | GPR | APRI | FIB-4 |
| Fibrosis grade | ||||
| F1 | 122 | 0.54 ± 1.23 | 53.18 ± 134.37 | 331.04 ± 1152.78 |
| r | 0.077 | 0.089 | 0.077 | |
| P | < 0.01 | < 0.01 | < 0.01 | |
| F2 | 52 | 0.66 ± 2.02 | 47.55 ± 120.04 | 278.98 ± 1053.17 |
| r | 0.223 | 0.069 | 0.037 | |
| P | < 0.01 | < 0.01 | < 0.01 | |
| F3 | 61 | 0.60 ± 0.95 | 61.15 ± 152.26 | 406.94 ± 1580.52 |
| r | 0.278 | 0.226 | 0.183 | |
| P | < 0.01 | < 0.01 | < 0.01 | |
| F4 | 41 | 0.52 ± 0.85 | 49.43 ± 125.04 | 287.50 ± 1092.13 |
| r | 0.186 | 0.121 | 0.064 | |
| P | < 0.01 | < 0.01 | < 0.01 |
ROC analysis of GPR, APRI, and FIB-4 in diagnosis of hepatic fibrosis in CHB
The AUC values for the GPR in F1, F2, F3, and F4 were 0.723, 0.741, 0.826, and 0.833, respectively, which were significantly higher than the values for the FIB-4 and APRI (F1: 0.581 and 0.612; F2: 0.706 and 0.711; F3: 0.73 and 0.751; and F4: 0.799 and 0.778). The diagnostic cut-off points for each stage were, respectively, 0.402, 0.448, 0.548, and 0.833. The diagnostic sensitivity and specificity had a gradually increasing trend with the aggravation of hepatic fibrosis. In particular, the diagnostic sensitivity and specificity were, respectively, 88.8% and 87.5% in F1, 72.7% and 89.7% in F2, 81.3% and 98.6% in F3, and 80% and 97.4% in F4 when GPR and APRI were connected in parallel; 86.6% and 90.2 %, 78.4% and 96%, 78.6% and 97.4%, and 73.2% and 97.9%, respectively, when GPR and APRI were connected in series; 80.2% and 89%, 65% and 89%, 70.3% and 98.5%, and 78.8% and 96.8%, respectively, when GPR and APRI were connected in parallel; and 83.6% and 87.9%, 76.8% and 96.6%, 72.7% and 98%, and 74.4% and 97.7%, respectively, when GPR and APRI were connected in series (Figure 1, Table 4).
Figure 1.
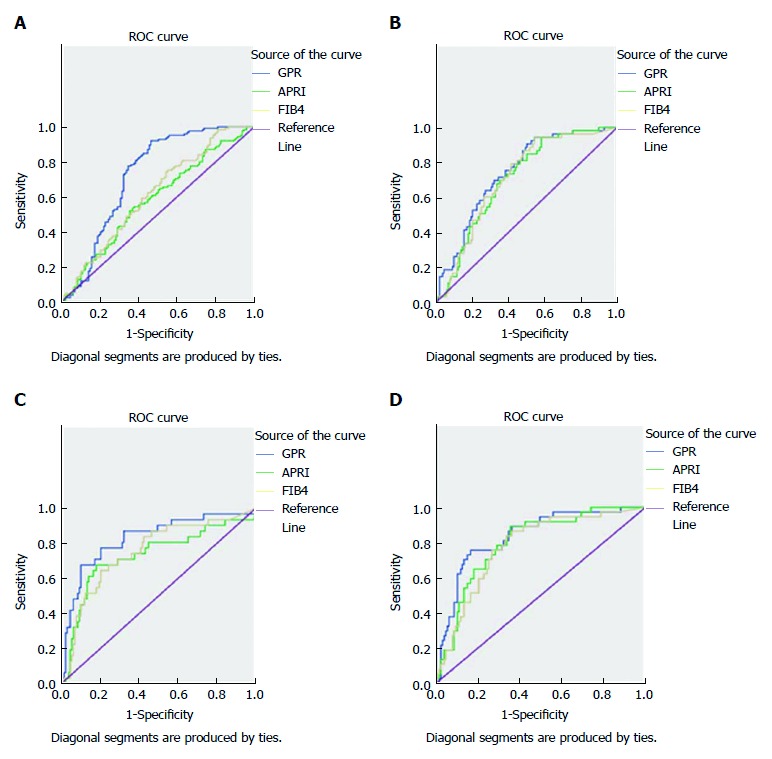
ROC curves of GPR, APRI, and FIB-4 for diagnosing liver fibrosis. A: F = 1; B: F = 2; F = 3; F = 4.
Table 4.
GPR, APRI, and FIB-4 results relative to hepatic fibrosis stage (F1, F2, F3, and F4) in CHB patients
| Parameter | GPR | APRI | FIB-4 | GPR + APRI (in parallel) | GPR + APRI (in series) | GPR + FIB-4 (in parallel) | GPR + FIB-4 (in series) | |
| AUC | 0.723 | 0.581 | 0.612 | - | - | - | - | |
| Cut-off | 0.448 | 0.166 | 0.201 | - | - | - | - | |
| F1 | Sensitivity | 90.6% | 88.8% | 62.1% | 88.8% | 86.8% | 80.2% | 83.6% |
| Specificity | 54.2% | 87.5% | 66.3% | 87.5% | 90.2% | 89% | 87.9% | |
| AUC | 0.741 | 0.706 | 0.711 | - | - | - | - | |
| Cut-off | 0.402 | 0.361 | 0.4 | - | - | - | - | |
| F2 | Sensitivity | 57.1% | 75.8% | 47.4% | 72.7% | 78.4% | 65% | 76.8% |
| Specificity | 89.5% | 92.5% | 89.3% | 89.7% | 96% | 89% | 96.6% | |
| AUC | 0.826 | 0.73 | 0.751 | - | - | - | - | |
| Cut-off | 0.548 | 0.496 | 0.44 | - | - | - | - | |
| F3 | Sensitivity | 74.1% | 70.6% | 64.7% | 81.3% | 78.6% | 70.3% | 72.7% |
| Specificity | 91.8% | 90.5% | 88% | 98.6% | 97.4% | 98.5% | 98% | |
| AUC | 0.833 | 0.799 | 0.778 | - | - | - | - | |
| Cut-off | 0.591 | 0.538 | 0.503 | - | - | - | - | |
| F4 | Sensitivity | 83.2% | 74% | 75.4% | 80% | 73.2% | 78.8% | 74.4% |
| Specificity | 96.5% | 95.4% | 94.5% | 97.4% | 97.9% | 96.8% | 97.7% |
In parallel: Both parameters are met simultaneously; In series: One of two parameters is met.
DISCUSSION
CHB is an inevitable stage for patients with HBV infection, developing from hepatic fibrosis to cirrhosis and leading to chronic liver failure and HBV-related hepatocellular carcinoma. Assessment of the degree of hepatic fibrosis and the progression of CHB is important in determining the treatment strategy for and prognosis of CHB patients[12,13]. Currently, liver biopsy is still the gold standard for evaluating the degree of hepatic fibrosis in certain high-risk liver cirrhosis and liver cancer patients and is an indicator for clinicians to guide patients, especially CHB patients with mildly elevated AIT, to start antiviral therapy in a timely fashion. However, as an invasive examination, liver biopsy can cause serious complications[14-18]; thus, it is not preferred by most CHB patients in the clinic. Given this situation, strategies for avoiding and reducing the frequency of liver biopsy and obtaining reliable liver pathological data are urgently needed[19-21]. The APRI and FIB-4 were developed as serum models in recent years and have been widely used in the assessment of hepatic fibrosis classification[22-26]. However, for patients with severe liver inflammation, the hepatic fibrosis results yielded by the APRI and FIB-4 are variable. Therefore, these indexes are predominantly used for hepatic fibrosis evaluation in patients with mildly abnormal liver function before treatment. Recently, Lemoine et al[3] analysed the combination of GGT and PLT count and found that the GPR was significantly better than the FIB-4 and APRI in predicting significant liver fibrosis. However, due to the sample size, the depth of the study was limited. To further explore the value of applying the GPR, a correlation analysis of 390 newly diagnosed CHB patients who were not treated with hepatoprotective therapy, anti-liver fibrosis drugs, or antiviral drugs to assess the correlation of the GPR, APRI, and FIB-4 with age, gender, medical history, liver function (TBil, ALT, AST), GGT, PLT count, and liver tissue fibrosis stage. The results showed that the GPR, APRI, and FIB-4 had no correlation with the CHB patients’ age, gender, or duration, but were correlated with TBil, AST, ALT, GGT, and PLT count. Furthermore, the GPR, APRI, and FIB-4 were positively associated with liver function and liver tissue pathological grade (P < 0.01). These results indicate that the GPR, as well as the APRI and FIB-4, can not only be used as an index to judge the severity of acute liver injury, but also has potential value in evaluating the degree of hepatic fibrosis in patients with CHB.
To further analyse the value of the GPR for the diagnosis of hepatic fibrosis, we performed ROC analysis of the GPR, APRI, and FIB-4 according to the stage of liver fibrosis. The results showed that the AUC values for GPR in F1, F2, F3, and F4 were 0.723, 0.741, 0.826 and 0.833, respectively, which were significantly better than the values for the APRI and FIB-4 (F1: 0.581 and 0.612; F2: 0.706 and 0.711; F3: 0.73 and 0.751; and F4: 0.799 and 0.778). The diagnostic cut-off points for each stage were, respectively, 0.402, 0.448, 0.548, and 0.833. The diagnostic sensitivity and specificity showed a gradually increasing trend with the aggravation of liver fibrosis. Thus, the combined detection of the GPR and FIB-4 or the GPR and APRI could improve the sensitivity and specificity the diagnosis of CHB-associated hepatic fibrosis in each stage. These results indicate that the GPR can accurately differentiate the stages of hepatic fibrosis in CHB patients, and that the diagnostic value of the GPR is superior to those of the APRI and FIB-4. Thus, the combined detection of the GPR and FIB-4 or the GPR and APRI can significantly improve the diagnostic sensitivity and specificity of hepatic fibrosis diagnosis in CHB. However, serum GGT levels may increase slightly to moderately in acute hepatitis, chronic active hepatitis, decompensated cirrhosis, obstructive jaundice, alcoholism, and hepatocellular carcinoma cases[16,27-28], which may lead to variation of the GPR. Due to the sample size in this study, we did not further stratify the cohort by liver function. The value of the GPR in evaluating the degree of hepatic fibrosis in CHB patients with different levels of liver function is thus still unknown. Further research involving stratified analyses and verification of our results with increased sample sizes is needed.
ARTICLE HIGHLIGHTS
Research background
Hepatic fibrosis is a precursor of cirrhosis in patients with chronic hepatitis B (CHB). Severe hepatic fibrosis and cirrhosis can increase the incidence and mortality of primary liver cancer. Looking for a non-invasive, simple, easy to operate, cheap way to assess liver fibrosis is very important.
Liver biopsy is still the gold standard for the diagnosis of liver fibrosis, but it is not widely used as a routine examination because of its invasiveness, high cost, and lack of repeatability. As an alternative, transient elastography (Fibroscan) by imaging is considered a good tool for the diagnosis of hepatic fibrosis, but its performance is restricted by several factors.
Researchers have tried to identify a simple, convenient, cheap, and noninvasive index to assess patients’ hepatic fibrosis level, which could assist in early diagnosis to achieve timely treatment, delay cirrhosis or liver cancer occurrence, improve patients’ quality of life, and prolong patients’ survival time.
Research motivation
At present, liver biopsy is still the gold standard for the diagnosis of liver fibrosis, but liver biopsy can cause serious complications; thus, it is not preferred by most CHB patients in the clinic. Our study aimed to identify a simple, convenient, cheap, and noninvasive index to assess patients’ hepatic fibrosis level.
Research objectives
Our study aimed to identify a simple, convenient, cheap, and noninvasive index to assess patients’ hepatic fibrosis level. In the future, in our clinical work, assessing the level of liver fibrosis in patients is faster and easier, effectively avoiding the complications caused by liver biopsy.
Research methods
There were 652 outpatients and inpatients diagnosed with CHB at the General Hospital of Ningxia Medical University. Patients with severe heart, brain or kidney disease; severe infection; superinfection with hepatitis A virus, hepatitis C virus, hepatitis D virus, or hepatitis E virus or autoimmune liver disease; heavy drinking; or pregnancy were excluded. The remaining 390 newly diagnosed CHB patients, who did not undergo hepatoprotective, anti-hepatic fibrosis drug, or antiviral drug treatments, were selected for the study. We chose multiple noninvasive indicators for comparison, to illustrate that GPR is an optimal noninvasive index. Meanwhile, the sample size was large to enhance persuasiveness. Statistically analysed using SPSS17.0 software, the data are expressed as the mean ± SD. Normal distributed data were compared by t-test, and non-normally distributed data were compared using the Chi-square test. In addition, correlations were determined by Pearson correlation analysis; P < 0.05 indicated that the difference was statistically significant.
Research results
The GPR is a simple, convenient, cheap, and noninvasive index to assess patients’ hepatic fibrosis level, and it can significantly improve the sensitivity and specificity of hepatic fibrosis diagnosis in CHB when combined with the FIB-4 or APRI.
Research conclusions
The GPR is a newly reported model for evaluating the grade of hepatic fibrosis, which is of great value in predicting hepatic fibrosis. In June 2015, Lemoine et al first reported that the GPR could be widely used as an independent predictor to assess hepatic fibrosis in CHB patients in West Africa, and that the sensitivity of the GPR is higher than those of the APRI and FIB-4. GPR is a simple, convenient, cheap, and noninvasive index to assess patients’ hepatic fibrosis level, and it can significantly improve the sensitivity and specificity of hepatic fibrosis diagnosis in CHB when combined with the FIB-4 or APRI. In the future, in our clinical work, assessing the level of liver fibrosis in patients is faster and easier, effectively avoiding the complications caused by liver biopsy.
Research perspectives
In the future work, it is required to look for more noninvasive, convenient and efficient methods to assess the level of liver fibrosis in patients with chronic hepatitis B.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): D, D
Grade E (Poor): 0
Supported by National Natural Science Foundation of China, No. 81460301 and No. 81760363; Key Project of Natural Science Foundation of Ningxia, No. NZ15134.
Conflict-of-interest statement: The authors declare that there are no conflicts of interest related to this study.
Data sharing statement: No additional data are available.
Peer-review started: August 7, 2017
First decision: September 4, 2017
Article in press: September 28, 2017
P- Reviewer: Sirin G, Jin B, He ST, Komatsu H, Koch TR, Ikura Y S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Huang Y
Contributor Information
Yan-Chao Hu, Department of Infectious Diseases, General Hospital of Ningxia Medical University, Yinchuan 750001, Ningxia Hui Autonomous Region, China.
Hao Liu, Department of Gastroenterology, General Hospital of Ningxia Medical University, Yinchuan 750001, Ningxia Hui Autonomous Region, China.
Xiao-Yan Liu, Department of Infectious Diseases, General Hospital of Ningxia Medical University, Yinchuan 750001, Ningxia Hui Autonomous Region, China.
Li-Na Ma, Department of Infectious Diseases, General Hospital of Ningxia Medical University, Yinchuan 750001, Ningxia Hui Autonomous Region, China.
Yu-Hua Guan, Department of Infectious Diseases, General Hospital of Ningxia Medical University, Yinchuan 750001, Ningxia Hui Autonomous Region, China.
Xia Luo, Department of Infectious Diseases, General Hospital of Ningxia Medical University, Yinchuan 750001, Ningxia Hui Autonomous Region, China.
Xiang-Chun Ding, Department of Infectious Diseases, General Hospital of Ningxia Medical University, Yinchuan 750001, Ningxia Hui Autonomous Region, China. dingxiangchun@nyfy.com.cn.
References
- 1.Liang P, Zu J, Yin J, Li H, Gao L, Cui F, Wang F, Liang X, Zhuang G. The independent impact of newborn hepatitis B vaccination on reducing HBV prevalence in China, 1992-2006: A mathematical model analysis. J Theor Biol. 2015;386:115–121. doi: 10.1016/j.jtbi.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 2.Wu JF, Chang MH. Natural history of chronic hepatitis B virus infection from infancy to adult life - the mechanism of inflammation triggering and long-term impacts. J Biomed Sci. 2015;22:92. doi: 10.1186/s12929-015-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lemoine M, Shimakawa Y, Nayagam S, Khalil M, Suso P, Lloyd J, Goldin R, Njai HF, Ndow G, Taal M, et al. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 2016;65:1369–1376. doi: 10.1136/gutjnl-2015-309260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemoine M, Thursz M, Mallet V, Shimakawa Y. Diagnostic accuracy of the gamma-glutamyl transpeptidase to platelet ratio (GPR) using transient elastography as a reference. Gut. 2017;66:195–196. doi: 10.1136/gutjnl-2016-311554. [DOI] [PubMed] [Google Scholar]
- 5.Park YE, Kim BK, Park JY, Kim DY, Ahn SH, Han KH, Han S, Jeon MY, Heo JY, Song K, et al. Gamma-glutamyl transpeptidase-to-platelet ratio is an independent predictor of hepatitis B virus-related liver cancer. J Gastroenterol Hepatol. 2017;32:1221–1229. doi: 10.1111/jgh.13653. [DOI] [PubMed] [Google Scholar]
- 6.Wang WL, Zheng XL, Zhang ZY, Zhou Y, Hao J, Tang G, Li O, Xiang JX, Wu Z, Wang B. Preoperative γ-glutamyl transpeptidase to platelet ratio (GPR) is an independent prognostic factor for HBV-related hepatocellular carcinoma after curative hepatic resection. Medicine (Baltimore) 2016;95:27 (e4087). doi: 10.1097/MD.0000000000004087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pang Q, Bi JB, Wang ZX, Xu XS, Qu K, Miao RC, Chen W, Zhou YY, Liu C. Simple models based on gamma-glutamyl transpeptidase and platelets for predicting survival in hepatitis B-associated hepatocellular carcinoma. Onco Targets Ther. 2016;9:2099–2109. doi: 10.2147/OTT.S101465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Song J, Huang Y, Li X, Zhuo Q, Li W, Chen C, Lu C, Qi X, Chen L. The Gamma-Glutamyl-Transpeptidase to Platelet Ratio Does not Show Advantages than APRI and Fib-4 in Diagnosing Significant Fibrosis and Cirrhosis in Patients With Chronic Hepatitis B: A Retrospective Cohort Study in China. Medicine (Baltimore) 2016;95:e3372. doi: 10.1097/MD.0000000000003372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyd A, Bottero J, Lacombe K. Theγ-glutamyltranspeptidase-to-plateletratio as a predictor of liverfibrosisinpatients co-infectedwith HBV and HIV. Gut. 2016;65:718–720. doi: 10.1136/gutjnl-2015-310607. [DOI] [PubMed] [Google Scholar]
- 10.Schiavon LL, Narciso-Schiavon JL, Ferraz MLG, Silva AEB, Carvalho-Filho RJ. The γ-glutamyl transpeptidase to platelet ratio (GPR) in HBV patients: just adding up? Gut. 2017;66:1169–1170. doi: 10.1136/gutjnl-2016-312658. [DOI] [PubMed] [Google Scholar]
- 11.Li Q, Lu C, Li W, Huang Y, Chen L. Globulin-platelet model predicts significant fibrosis and cirrhosis in CHB patients with high HBV DNA and mildly elevated alanine transaminase levels. Clin Exp Med. 2017 doi: 10.1007/s10238-017-0472-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 13.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 14.Sorbi D, Boynton J, Lindor KD. The ratio of aspartate aminotransferase to alanine aminotransferase: potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am J Gastroenterol. 1999;94:1018–1022. doi: 10.1111/j.1572-0241.1999.01006.x. [DOI] [PubMed] [Google Scholar]
- 15.McGill DB, Rakela J, Zinsmeister AR, Ott BJ. A 21-year experience with major hemorrhage after percutaneous liver biopsy. Gastroenterology. 1990;99:1396–1400. doi: 10.1016/0016-5085(90)91167-5. [DOI] [PubMed] [Google Scholar]
- 16.Shukla A, Kapileswar S, Gogtay N, Joshi A, Dhore P, Shah C, Abraham P, Bhatia S. Simple biochemical parameters and a novel score correlate with absence of fibrosis in patients with nonalcoholic fatty liver disease. Indian J Gastroenterol. 2015;34:281–285. doi: 10.1007/s12664-015-0580-5. [DOI] [PubMed] [Google Scholar]
- 17.Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S; Chronic Hepatitis B Guideline Working Party of the Asian-Pacific Association for the Study of the Liver. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263–283. doi: 10.1007/s12072-008-9080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Liu C, Chen H, Liu Q, Yang B, Ou Q. Study on noninvasive laboratory tests for fibrosis in chronic HBV infection and their evaluation. J Clin Lab Anal. 2013;27:5–11. doi: 10.1002/jcla.21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee IC, Chan CC, Huang YH, Huo TI, Chu CJ, Lai CR, Lee PC, Su CW, Hung HH, Wu JC, et al. Comparative analysis of noninvasive models to predict early liver fibrosis in hepatitis B e Antigen-negative Chronic Hepatitis B. J Clin Gastroenterol. 2011;45:278–285. doi: 10.1097/MCG.0b013e3181dd5357. [DOI] [PubMed] [Google Scholar]
- 21.Pinto J, Matos H, Nobre S, Cipriano MA, Marques M, Pereira JM, Gonçalves I, Noruegas MJ. Comparison of acoustic radiation force impulse/serum noninvasive markers for fibrosis prediction in liver transplant. J Pediatr Gastroenterol Nutr. 2014;58:382–386. doi: 10.1097/MPG.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 22.Sebastiani G, Halfon P, Castera L, Mangia A, Di Marco V, Pirisi M, Voiculescu M, Bourliere M, Alberti A. Comparison of three algorithms of non-invasive markers of fibrosis in chronic hepatitis C. Aliment Pharmacol Ther. 2012;35:92–104. doi: 10.1111/j.1365-2036.2011.04897.x. [DOI] [PubMed] [Google Scholar]
- 23.Arora A, Sharma P. Non-invasive Diagnosis of Fibrosis in Non-alcoholic Fatty Liver Disease. J Clin Exp Hepatol. 2012;2:145–155. doi: 10.1016/S0973-6883(12)60103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology. 2015;61:292–302. doi: 10.1002/hep.27382. [DOI] [PubMed] [Google Scholar]
- 25.Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono M, Fujii H, Eguchi Y, Suzuki Y, Aoki N, Kanemasa K, Fujita K, Chayama K, Saibara T, Kawada N, Fujimoto K, Kohgo Y, Yoshikawa T, Okanoue T; Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG-NAFLD) Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol. 2012;12:2. doi: 10.1186/1471-230X-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elawdi HA, Franzini M, Paolicchi A, Emdin M, Fornaciari I, Fierabracci V, De Simone P, Carrai P, Filipponi F. Circulating gamma-glutamyltransferase fractions in cirrhosis. Liver Int. 2014;34:e191–e199. doi: 10.1111/liv.12455. [DOI] [PubMed] [Google Scholar]
- 28.Eminler AT, Irak K, Ayyildiz T, Keskin M, Kiyici M, Gurel S, Gulten M, Dolar E, Nak SG. The relation between liver histopathology and GGT levels in viral hepatitis: more important in hepatitis B. Turk J Gastroenterol. 2014;25:411–415. doi: 10.5152/tjg.2014.3693. [DOI] [PubMed] [Google Scholar]


