Figure 2. DOK3 is required to limit osteoclast differentiation and function.
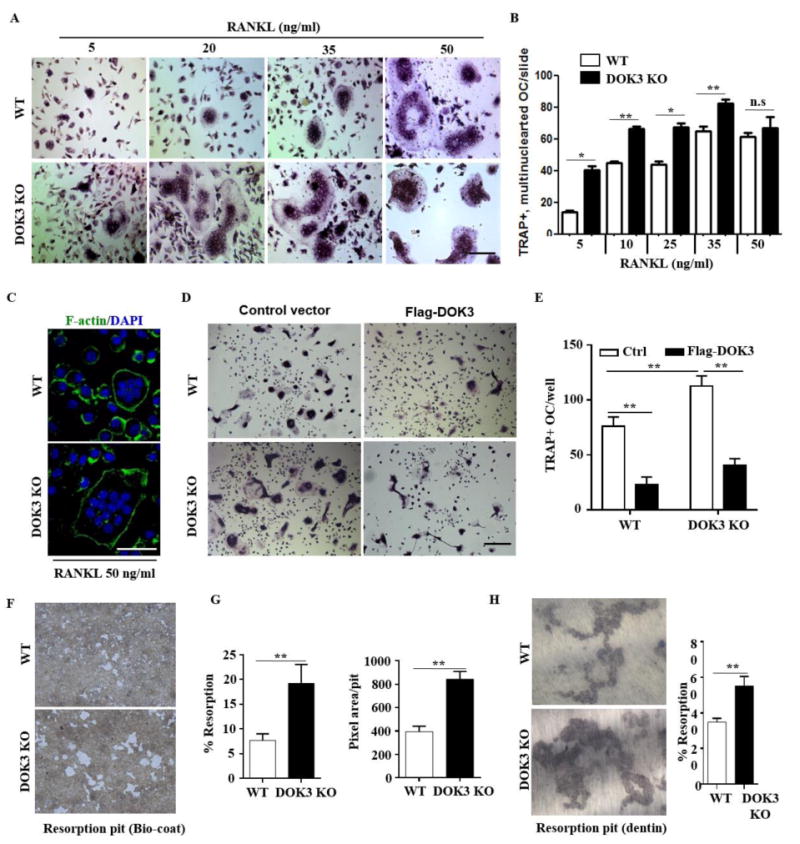
(A and B) BMMs were proliferated for 2 days 20 ng/ml M-CSF and equal numbers of non-adherent pre-osteoclasts were plated and differentiation was induced with 20 ng/ml M-CSF and increasing concentrations of RANKL 5-50 ng/ml for 5 days. Cells were fixed and stained for TRAP. Representative images were captured by light microscopy (A) and TRAP+ multinucleated (≥3 nuclei) osteoclasts quantified (B). (C) Osteoclasts derived in the presence of 20 ng/ml M-CSF and 50 ng/ml RANKL were fixed and permeabilized followed by staining with Alexa Fluor® 488 phalloidin to visualize actin rings. (D and E) WT or DOK3-/- BMMs were transfected with control or Flag-DOK3 expression plasmids and differentiated with 20 ng/ml M-CSF and 50 ng/ml RANKL on tissue culture plastic. Cells were fixed and stained for TRAP. Representative images were captured by light microscopy (D) and TRAP-positive stained cells (≥ 3 nuclei) were quantified as osteoclasts (E). (F-H) WT or DOK3-/- BMMs were plated and differentiated with RANKL 50 ng/ml and M-CSF 20 ng/ml for 3 days, lifted and plated in equal numbers onto BD Biocoat calcium-phosphate substrate and treated with RANKL 50 ng/ml and M-CSF 20 ng/ml for 5 days or 10 days for dentin slices. (F) Representative images of resorption pits (white areas) and (G) percent resorption (%) and pixel area per pit were quantified (n=∼500 pits per group). (H) Representative images of resorption pits were captured and percent resorption (%) was quantified. Scale bar: 100 μm (A and D) and 50 μm (C). Experiments was repeated three times. Statistical values were calculated using a Student's t-test unless otherwise indicated, *p < 0.05, **p < 0.01, n.s: no significance. Error bars indicate the mean ± SD of triplicates.
